Since 2015, when the US Food and Drug Administration (FDA) first approved nivolumab to treat patients with advanced non-small cell lung cancer (NSCLC), clinicians have witnessed an improvement in overall survival (OS) rates using anti-PD-(L)1 agents to inhibit cancer cell growth in both the first- and second-line setting.1
Is this decade-long reign coming to an end? Will targeted bispecific antibodies replace PD-(L)1 agents as the primary immunotherapy for lung cancer?
Two experts in thoracic oncology debated this question on February 20 during the IASLC Targeted Therapies of Lung Cancer Meeting, which registered attendees can watch on demand via Lung Cancer 360.

Jonathan W. Riess, MD, MS, Professor of Medicine and Director of Thoracic Oncology at UC Davis Comprehensive Cancer Center, defended the position that bispecific antibodies are poised to take the immunotherapy crown from PD-(L) agents.
His arguments focused on three key points:
- Bispecific antibodies present opportunities to target multiple components of the cancer immunity cycle including PD-(L)1 blockade.
- Bispecific antibodies offer enhanced mechanistic advantages, such as cooperative binding activity.
- Bispecific antibodies have exhibited promising initial data.
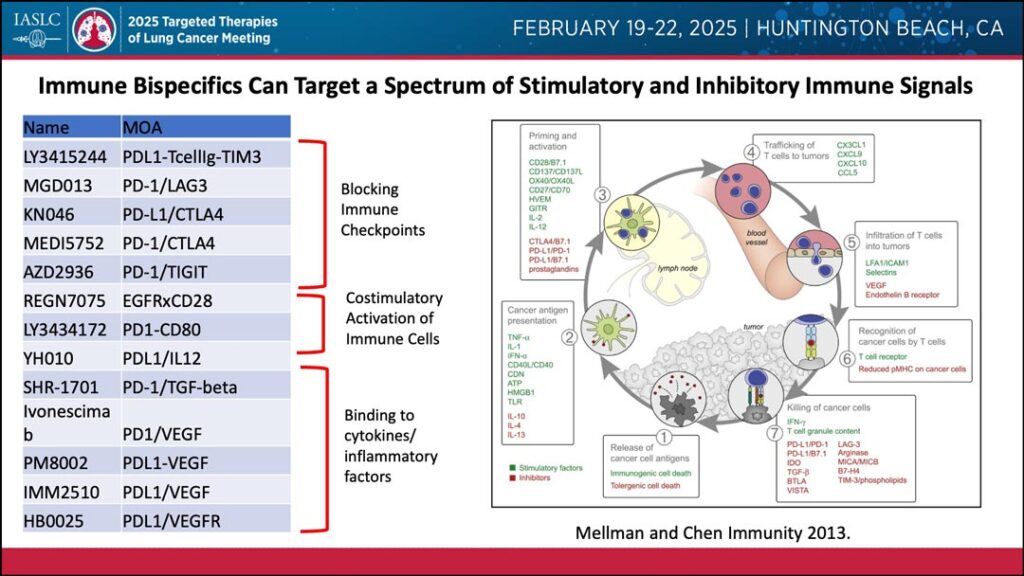
Dr. Riess noted that bispecific antibodies have a unique ability for combination attacks across a spectrum of stimulatory and inhibitory immune signals, including blocking immune checkpoints, costimulatory activation of immune cells, and binding to cytokines and inflammatory factors.
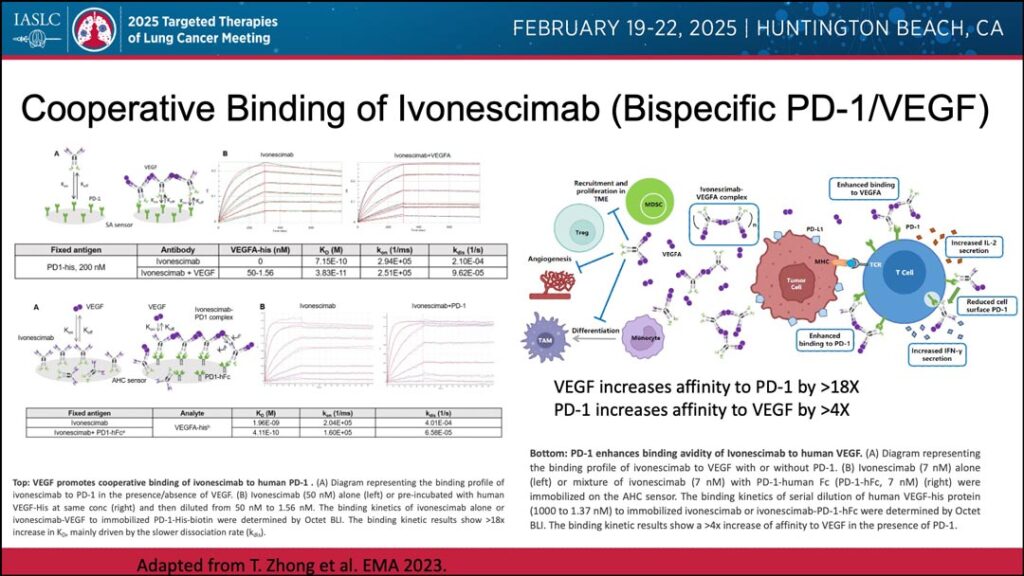
Further, Dr. Riess noted that bispecific antibodies have shown mechanistic advantages, such as cooperative binding. This has been observed with ivonescimab, a bispecific antibody that targets both PD-1 and VEGF.
As an example, Riess pointed to work indicating that bispecific cooperative bindings of ivonescimab with VEGF can increase PD-1 affinity by more than 18 times, while cooperative bindings of ivonescimab and PD-1 can increase VEGF affinity by more than four times.2
Evaluating clinical results, Dr. Riess singled out the recent randomized phase III HARMONi-2 study in PD-L1 positive NSCLC, which featured a head-to-head comparison of bispecific ivonescimab against the single agent monoclonal antibody, pembrolizumab.3
“The results showed a substantial and statistically significant progression-free survival (PFS) benefit,” said Dr. Riess, adding that the benefit of ivonescimab was seen in patients with both low and high PD-L1 expression.

Dr. Riess characterized the initial studies of bispecific antibodies in EGFR-mutated NSCLC as “very promising” and noted that recent data on other bispecific antibodies, such as volrustomig, with favorable PFS data in the harder to treat PD-L1 negative population.4
Greg Kalemkerian, MD, Professor of Medicine at the University of Michigan, approached the issue from a different perspective and dismissed what he characterized as unproven assumptions regarding bispecific antibodies.
“Why would bispecific antibodies be better than combination therapy? We’ve heard a lot of preliminary data and some biological rationale, but it really needs to be clarified,” Dr. Kalemkerian said. “I think the crux of this comes down to the question: Does the bispecific immunological targeting improve survival over single-agent immune checkpoint inhibitor therapy?”
Dr. Kalemkerian countered Dr. Riess’s presentation by presenting a series of studies that did not prove benefit of adding immune checkpoint or VEGF targeting agents to anti PD-1/PD-L1 therapy. He noted that the string of unsuccessful studies involving more than 7,000 patients.
He reviewed survival data from trials using angiogenesis targeting bevacizumab and multitargeted tyrosine kinase inhibitors including lenvatinib, cabozantinib, and sitravatinib. In discussing the Lung-MAP S1800A,5 he noted lack of progression-free survival and response benefit with pembrolizumab and ramucirumab in patients that had been treated with anti-PD-1/anti-PD-L1 therapy, though this trial had overall survival as the primary endpoint, which met statistical significance.
Other negative trials included the APPLE (WJOG) trial,6 which found no meaningful benefit to adding anti-angiogenetic bevacizumab to chemotherapy, the LEAP-007 trial,7 which revealed that a first-line treatment of lenvatinib (anti-angiogenesis) with pembrolizumab (anti-PD-L1) was less effective than pembrolizumab alone with more toxicities.
Additionally, the SAPPHIRE trial8 demonstrated that adding an anti-VEGFR therapy to immunotherapy does not improve the results when comparing a combination of sitravatinib and nivolumab to docetaxel monotherapy in the 2nd line setting.
Turning to the HARMONi-2 phase III trial, Dr. Kalemkerian conceded that the results appeared to be positive for the bispecific ivonescimab over pembrolizumab. However, he pointed out significant factors to consider.
“There are no overall survival data; single-agent pembrolizumab is not really the standard of care for patients with PD-L1 expression of 1-49%; median follow-up remains short; and we await broader geographic and ethnic confirmation of this trial,” he said.
The results of studies focused on combinations of anti-PD-(L)1 plus anti-CTLA-4 agents were thought equally unconvincing in both non-small and small cell lung cancer, according to Dr. Kalemkerian. Going through a series of studies from 2019 to 2023, he found results ranging from “no meaningful benefit” to “increased toxicity.”
Some examples provided were “lose lose,” such as the ARCTIC trial, which compared durvalumab plus tremelimumab, versus durvalumab monotherapy, and SOC chemotherapy as a third-line plus therapy for NSCLC with PD-L1 expressions greater than 25%.
The trial found there was more toxicity with the combination of PD-L1 and CTLA-4, with treatments-related adverse events (TRAEs) between grade 3-4 in 22% of the combination group versus 12% for durvalumab monotherapy. Moreover, there was no OS benefit: median OS was 11.5 months for the combination vs 10 months for durvalumab alone.
Shifting into small-cell lung cancer (SCLC), Dr. Kalemkerian pointed to the CASPIAN trial, which examined the efficacy of durvalumab with tremelimumab versus durvalumab alone, as well as placebo.
The combination of durvalumab with tremelimumab was less effective in terms of OS, with a median OS of 10.4 months versus 12.9 months for durvalumab alone. Additionally, the combination therapy was associated with higher toxicity rates; 10% of patients experienced grade 5 TRAEs compared with 5% in the durvalumab-only group.
Overall, “we have a lot of failed ICI-plus-anything-other-than-chemotherapy strategies, and I promise you there will be more coming,” he said. “So, will immunotherapy bispecific antibodies replace PD-(L)1 agents? The answer is ‘no.’ At least not today.”
And with that “at least not today,” Dr. Kalemkerian echoed a key concept voiced by his debate opponent—timing.
Do we cast the verdict on bispecific antibodies based on what we know today, or do we base this verdict on what we think we will soon discover?
In his presentation, Dr. Riess approached the question by looking at the near future and invoking the wisdom of hockey legend Wayne Gretzky—to skate to where the puck is going, not to where it has been.9
In turn, Dr. Kalemkerian seemed to be asking colleagues to evaluate data based on the most effective treatment today and to ensure they had their eyes on the right puck before skating off into what could be the wrong corner.
References
- 1. Parvez A, Choudhary F, Mudgal P, Khan R, Qureshi KA, Farooqi H, Aspatwar A. PD-1 and PD-L1: architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front Immunol. 2023 Dec 1;14:1296341. doi: 10.3389/fimmu.2023.1296341. PMID: 38106415; PMCID: PMC10722272.
- 2. Zhong T, et al. AACR-NCI-EORTC International Conference 2023. Poster #B123, Abstract #35333, Boston, MA, USA
- 3. Zhou C, Chen J, Wu L, et al: Phase 3 study of ivonescimab (AK112) vs pembrolizumab as first-line treatment for PD-L1–positive advanced NSCLC: Primary analysis of HARMONi-2. 2024 World Conference on Lung Cancer. Abstract PL02.04. Presented September 8, 2024.
- 4. Dovedi SJ, Elder MJ, Yang C, Sitnikova SI, Irving L, Hansen A, Hair J, Jones DC, Hasani S, Wang B, Im SA, Tran B, Subramaniam DS, Gainer SD, Vashisht K, Lewis A, Jin X, Kentner S, Mulgrew K, Wang Y, Overstreet MG, Dodgson J, Wu Y, Palazon A, Morrow M, Rainey GJ, Browne GJ, Neal F, Murray TV, Toloczko AD, Dall’Acqua W, Achour I, Freeman DJ, Wilkinson RW, Mazor Y. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1+ Activated T Cells. Cancer Discov. 2021 May;11(5):1100-1117. doi: 10.1158/2159-8290.CD-20-1445. Epub 2021 Jan 8. PMID: 33419761.
- 5. Reckamp KL, Redman MW, Dragnev KH, et al. Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non-Small-Cell Lung Cancer Previously Treated With Immunotherapy-Lung-MAP S1800A [published correction appears in J Clin Oncol. 2022 Sep 1;40(25):3002. doi: 10.1200/JCO.22.01464.]. J Clin Oncol. 2022;40(21):2295-2306. doi:10.1200/JCO.22.00912
- 6. Shiraishi Y, Kishimoto J, Tanaka K, Sugawara S, Daga H, Hirano K, Azuma K, Hataji O, Hayashi H, Tachihara M, Mitsudomi T, Seto T, Nakagawa K, Yamamoto N, Okamoto I. Treatment Rationale and Design for APPLE (WJOG11218L): A Multicenter, Open-Label, Randomized Phase 3 Study of Atezolizumab and Platinum/Pemetrexed With or Without Bevacizumab for Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2020 Sep;21(5):472-476. doi: 10.1016/j.cllc.2020.03.010. Epub 2020 Apr 10. PMID: 32381420.
- 7. Yang, James Chih-Hsin et al. Journal of Thoracic Oncology, Volume 19, Issue 6, 941 – 953 Pembrolizumab With or Without Lenvatinib for First-Line Metastatic NSCLC With Programmed Cell Death-Ligand 1 Tumor Proportion Score of at least 1% (LEAP-007): A Randomized, Double-Blind, Phase 3 Trial
- 8. Borghaei H, de Marinis F, Dumoulin D, Reynolds C, Theelen WSME, Percent I, Gutierrez Calderon V, Johnson ML, Madroszyk-Flandin A, Garon EB, He K, Planchard D, Reck M, Popat S, Herbst RS, Leal TA, Shazer RL, Yan X, Harrigan R, Peters S; SAPPHIRE Investigators. SAPPHIRE: phase III study of sitravatinib plus nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2024 Jan;35(1):66-76. doi: 10.1016/j.annonc.2023.10.004. Epub 2023 Oct 20. PMID: 37866811.
- 9. A common misattribution of a quote that may have come from Wayne Gretzky’s father