Lung cancer is the leading cause of cancer mortality worldwide, with an estimated 2.1 million new cases and 1.8 million deaths in 2018—more than colon, breast, and prostate cancers combined.1
SCLC is a high-grade neuroendocrine carcinoma, typically metastatic at diagnosis; it has an exceptionally poor prognosis, with a dismal median OS of approximately 1 year.2
,3
Annually, SCLC causes approximately one-quarter of a million deaths globally, including approximately 30,000 deaths in the United States.2
,3
For decades, the only systemic treatment option for this disease has been chemotherapy. Though it is initially effective, resistance to chemotherapy develops almost universally, and most SCLC tumors recur or progress. The main hurdles to finding an effective and sustainable treatment option for SCLC include an incomplete understanding of the biology of the disease, a lack of actionable targets, and the ineffectiveness of or rapid resistance to existing therapies, including immunotherapy.
Immune checkpoint inhibitors have been at the forefront of clinical practice for several types of solid tumors since the first of these drugs received approval in 2011. In many malignancies, tumor mutational burden has been utilized as a predictor of response to immunotherapy. Unfortunately, despite having one of the highest mutational burdens, SCLC has relatively limited sensitivity to immune checkpoint blockade (ICB). One particularly understudied feature of SCLC is its relatively immunosuppressed phenotype, which is characterized by low levels of infiltrating T cells and reduced antigen presentation. Reflecting the lack of effective immune engagement, ICB is only effective for a small subset of patients with SCLC, regardless of whether the regimen targets the PD-1/PD-L1 axis alone or is combined with an anti–CTLA-4 agent. ICB plus chemotherapy is FDA approved for the frontline treatment of SCLC on the basis of results of phase III IMpower133 trial, which demonstrated modest increases in OS with the addition of the anti–PD-L1 drug atezolizumab to chemotherapy.4
,5
More recently, results from the CASPIAN trial led to FDA approval of another anti–PD-L1 antibody, durvalumab, on the basis of a similarly modest survival benefit.6
,7
However, the survival benefits observed in these trials were modest, at best. The disappointing efficacy of immunotherapy illustrates the recalcitrance of SCLC and the need for novel treatment targets.
SCLC is characterized by dysregulation of genomic integrity and the cell cycle via biallelic inactivation of TP53 and RB1 (Fig. 1A). In exploring the biology of SCLC, we found higher levels of DNA damage response (DDR) proteins in preclinical models and clinical samples compared with NSCLC. In particular, our work demonstrated that SCLC cell lines and patient samples have increased expression of the crucial DDR proteins checkpoint kinase 1 (CHK1), ataxia telangiectasia and Rad3-related (ATR), and WEE1, relative to NSCLC (Fig. 1A). We demonstrated that a novel second-generation CHK1 inhibitor, prexasertib, has remarkable single-agent activity in vitro and in spontaneous genetically engineered mouse models of SCLC. Prexasertib significantly augmented the antitumor effects of cisplatin and overcame acquired chemotherapy resistance in SCLC in vitro and in vivo. The results of this study have led directly to the first-ever phase II clinical trial (NCT02735980) of a CHK1 inhibitor for extensive-stage SCLC. We further identified biomarkers that can predict response to CHK1 inhibitors, including MYC protein overexpression in SCLC cell lines. MYC amplification is a hallmark of SCLC, but small-molecule inhibitors of MYC are clinically ineffective. Hence, targeting MYC indirectly may be the best approach. We provided compelling preclinical evidence that CHK1 targeting could be a viable synthetic, lethal strategy in MYC-driven SCLC tumors.
Previous studies have shown that increased genomic instability predicts improved response to ICB. So, we next aimed to determine the effect of DDR targeting on the antitumor immune response in SCLC models. We treated a panel of human SCLC cell lines with either prexasertib, the PARP inhibitor olaparib, or genetic knockdown of these targets, and found that DDR targeting (genetic or pharmacologic) significantly increased the protein and surface expression of a primary ICB target, PD-L1, in all cell lines (Fig. 1B). We next evaluated the ability of DDR inhibition to sensitize tumors to PD-L1 blockade. In a genetically engineered SCLC mouse model, consistent with the patient response, mice treated with anti–PD-L1 alone showed no antitumor response. In contrast, DDR inhibition caused a significant delay in tumor growth, and the combination of a PARP inhibitor or a CHK1 inhibitor with an anti–PD-L1 agent led to dramatic and rapid tumor regression. The majority of the animals treated with the combination therapy had complete responses, and the tumors became undetectable by the end of the experiment (Fig. 1C). These encouraging data led us to test the combination treatment in additional preclinical models, which yielded similar responses. Concordant with the antitumor response, we observed significant increases in cancer-fighting CD8+ cytotoxic T cells in the tumors treated with the combination of a DDR inhibitor and immunotherapy. Furthermore, combined DDR inhibitor and ICB treatment decreased exhausted T cells and T regulatory cells (Fig. 1D). Thus, DDR inhibition in combination with ICB rescued the immune response in the mice and facilitated immune-mediated cancer killing. This was further confirmed when depletion of CD8+ T cells reversed the antitumor response of the DDR inhibitor and PD-L1 blockade.
Building on this success, we sought to delineate the underlying mechanism by which DDR targeting modulated the immune microenvironment in SCLC. One of the ways immune cells detect infections is by searching for fragments of cytoplasmic DNA, possibly from a virus or bacteria, that activate the innate immune system. We noticed that CHK1 or PARP inhibition led to increased cytoplasmic DNA in multiple SCLC models, which engaged the innate immune cGAS/STING pathway. As seen in multiple preclinical models of SCLC, activation of the cGAS/STING pathway led to increased expression of Type I interferon, IFNβ, which further led to induction of antitumorigenic chemokines CXCL10 and CCL5 (Fig. 1B). Therefore, we demonstrated that the STING pathway’s ability to respond to DNA damage could activate the immune system and drive an ICB response in these SCLC models.
Fig. 1. Model of DNA damage response (DDR) targeting to augment antitumor immune response in SCLC.
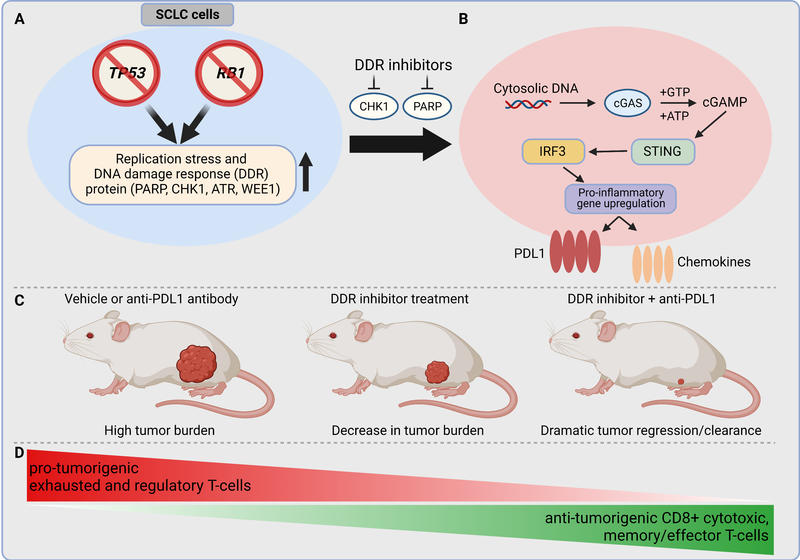
A: SCLC is characterized by almost ubiquitous loss-of-function mutation of the TP53 and RB1 genes. This leads to inherent genomic instability, increased replication stress, and upregulation of DDR pathway regulators. B: Targeting the DDR proteins PARP and checkpoint kinase 1 (CHK1) with the small-molecule inhibitors prexasertib and olaparib leads to cytosolic DNA in SCLC models. The cytosolic DNA is then recognized by cyclic GMP‐AMP synthase (cGAS), which leads to activation of the STING/TBK1/IRF3 pathway. IRF activation leads to increased expression of IFNβ and enhanced expression of the chemokines CXCL10 and CCL5. STING pathway activation and increased chemokine expression lead to increased PD-L1 expression in SCLC models. C: Treatment of flank tumors in immunocompetent mice with single-agent ICB is ineffective in causing tumor regression. Treatment with single-agent DDR inhibition causes decrease in tumor burden. However, treatment with the combination of DDR inhibition and PD-L1 blockade leads to dramatic synergy and, frequently, to complete, durable tumor regression. D: Combination therapy of a DDR inhibitor with PD-L1 blockade leads to changes in the immune tumor microenvironment by increasing antitumorigenic and decreasing protumorigenic immune cell subsets.
Abbreviations: ATP, adenosine triphosphate; ATR, taxia telangiectasia and Rad3-related; cGAMP, cyclic guanosine monophosphate–adenosine monophosphate (cyclic GMP-AMP); CTLA4, cytotoxic T-lymphocyte-associated protein 4; FDA, U.S. Food and Drug Administration; GTP, guanosine triphosphate; IRF3, interferon regulatory factor 3; STING, STimulator of INterferon Genes.
Taken together, we have demonstrated deep and durable preclinical responses with DDR inhibitors as monotherapy or when combined with ICB in SCLC. Our data have helped establish the role of DDR proteins as potentially effective therapeutic targets in SCLC and illustrated some important features of immuno-oncology research in SCLC. We defined the existence of multiple phases of immunosuppression in SCLC and explained why PD-L1 expression, unlike in other cancer types, has not proven to be a predictive biomarker in SCLC. Our study provides a compelling rationale for rapid translation to clinical trials testing combined DDR targeting with or without ICB in patients with SCLC and has already supported multiple clinical trials (Table). Given the aggressiveness of relapsed SCLC, the argument can be made about testing DDR inhibition and ICB in the maintenance setting to maximize tumor control. By translating this knowledge in the clinic in the coming years, we hope our work will have an impact on reducing morbidity and improving the quality of life of patients with SCLC and other malignancies harboring DDR defects.
| Trial Number | Study Title | interventions |
|---|---|---|
|
NCT02660034 |
The Safety, Pharmacokinetics and Antitumor Activity of BGB-A317 in Combination With BGB-290 in Participants with Advanced Solid Tumors |
Tislelizumab Pamiparib
|
| NCT02484404 | Phase I/II Study of the Anti-Programmed Death Ligand-1 Antibody MEDI4736 in Combination with Olaparib and/or Cediranib for Advanced Solid Tumors and Advanced or Recurrent Ovarian, Triple Negative Breast, Lung, Prostate and Colorectal Cancers | Olaparib Cediranib MEDI4736 |
| NCT04701307 | Niraparib and Dostarlimab for the Treatment of Small Cell Lung Cancer and Other High-Grade Neuroendocrine Carcinomas | Dostarlimab Niraparib |
| NCT03830918 | Niraparib, Temozolomide and Atezolizumab in Treating Patients with Advanced Solid Tumors and Extensive-Stage Small Cell Lung Cancer with a Complete or Partial Response to Platinum-Based First-Line Chemotherapy | Temozolomide Niraparib Atezolizumab |
| NCT04334941 | Testing Maintenance Therapy for Small Cell Lung Cancer in Patients with SLFN11 Positive Biomarker | Atezolizumab Talazoparib |
| NCT04538378 | Olaparib (LYNPARZA) Plus Durvalumab (IMFINZI) in EGFR-Mutated Adenocarcinomas That Transform to Small Cell Lung Cancer (SCLC) and Other Neuroendocrine Tumors | Olaparib Durvalumab |
| NCT03958045 | Combination Rucaparib With Nivolumab in Small Cell Lung Carcinoma | Rucaparib Nivolumab |
|
NCT02734004 |
A Phase I/II Study of MEDI4736 in Combination with Olaparib in Patients with Advanced Solid Tumors. (MEDIOLA) | Olaparib MEDI4736 Bevacizumab |
| NCT04782089 | Study of PD-1 Antibody and PARP Inhibitor in Extensive Stage Small Cell Lung Cancer | Camrelizumab Fluzoparib |
| NCT04728230 | Olaparib and Durvalumab With Carboplatin, Etoposide, and/or Radiation Therapy for the Treatment of Extensive-Stage Small Cell Lung Cancer, PRIO Trial | Carboplatin/etoposide Durvalumab Olaparib Radiation therapy |
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
- 2. a. b. Sen T, Gay CM, Byers LA. Targeting DNA damage repair in small cell lung cancer and the biomarker landscape. Transl Lung Cancer Res. 2018;7(1):50-68.
- 3. a. b. Taniguchi H, Sen T, Rudin CM. Targeted therapies and biomarkers in small cell lung cancer. Front Oncol. 2020. doi.org/10.3389/fonc.2020.00741
- 4. Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220-2229.
- 5. Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220-2229.
- 6. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939.
- 7. Paz-Ares L, Chen Y, Reinmuth N, et al. Overall survival with durvalumab plus etoposide-platinum in first-line extensive-stage SCLC: results from the CASPIAN study. J Thorac Oncol. 2019;14(10):S7-S8.










