Despite significant treatment advances, metastatic non-small cell lung cancer (mNSCLC) remains largely incurable. Most patients develop disease progression following first-line therapies, and there are limited treatment options to extend survival beyond progression.
So said investigator Ticiana Leal, MD, while presenting results from the phase III LUNAR study on June 6 at the American Society of Clinical Oncology’s 2023 Annual Meeting.
The LUNAR study evaluated the safety and efficacy of tumor treating fields (TTFields) with standard of care versus standard care alone in mNSCLC progressing on or after platinum-based therapy.
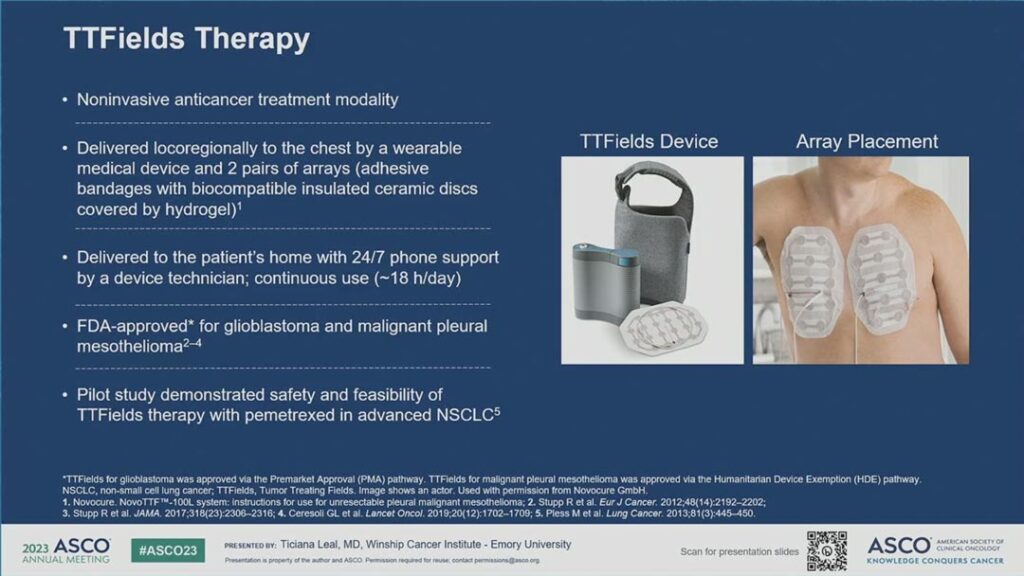
Dr. Leal described tumor treating fields as electric fields that exert physical forces on electrically charged intracellular components to disrupt mitosis, which in turn leads to aneuploidy and induction of endoplasmic reticulum stress. The downstream effects of this include immunogenic cell death, which triggers a systemic anti-tumor immune response. For the LUNAR study, the electric fields were generated by a portable medical device and delivered to the tumors through two pairs of arrays applied to the chest. (See Fig. 1)
Currently, TTFields therapy is approved for glioblastoma and malignant pleural mesothelioma. Previously, a pilot study demonstrated the safety and feasibility of TTFields therapy with pemetrexed in advanced NSCLC.
The LUNAR study included 276 adult patients with ECOG performance status of 0-2 randomized to TTFields therapy and standard of care—which included investigators’ choice of immune checkpoint inhibitor (ICI) or docetaxel—versus standard of care alone. Patients were followed every six weeks and continued therapy until progression or intolerable toxicities. The study enrolled patients at 124 sites in 17 countries, and the primary endpoint was overall survival in the study’s two primary arms.
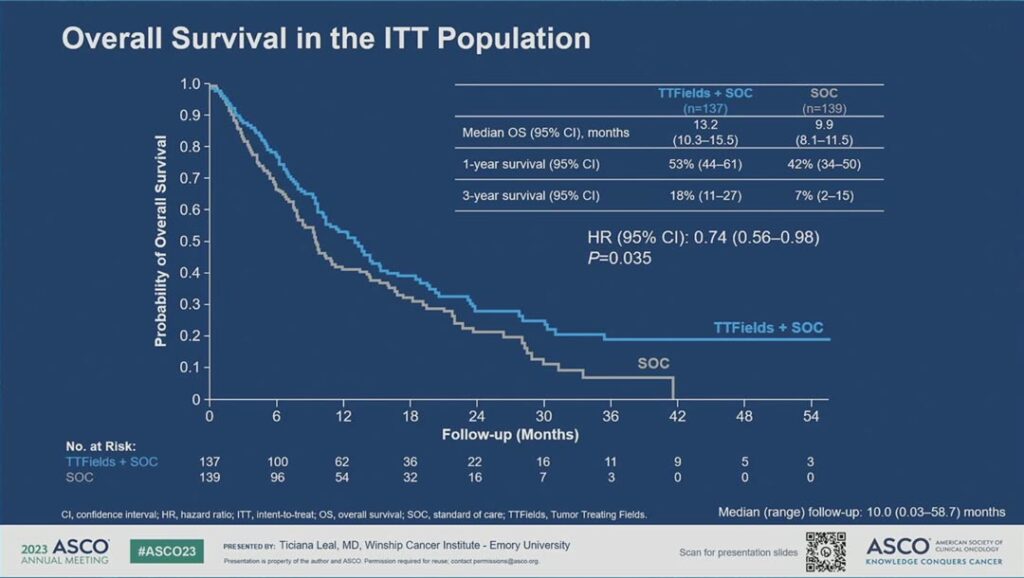
“The median overall survival in TTFields plus standard of care was 13.2 months versus 9.9 months in the standard of care with a hazard ratio of 0.74 and a p value of 0.035,” Dr. Leal said. “The curves separated early and maintained separation throughout. The 3-year survival rate was 18% in TTFields plus standard of care versus 7% in the standard of care arm.” (see Fig. 2)
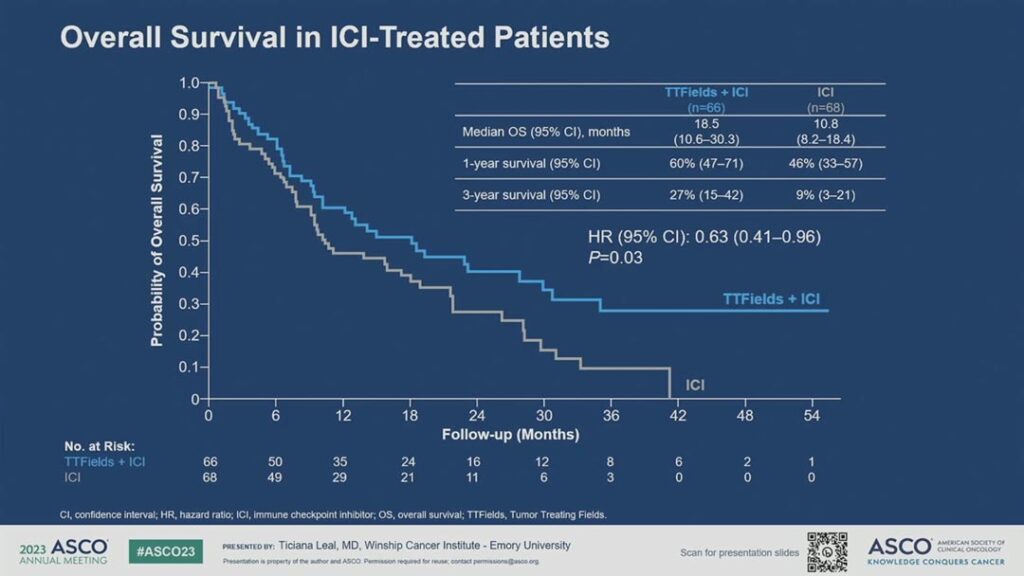
She said the overall survival benefit was particularly prominent in the ICI treated patients. The median overall survival was 18.5 months in the TTFields plus ICI subgroup versus 10.8 months in the ICI only subgroup. The 3-year survival rate was 27% versus 9%, respectively. (See Fig. 3) By comparison, the median overall survival in the TTFields plus docetaxel subgroup was 11.1 months versus 8.7 months in the docetaxel group.
In terms of safety, the only notable difference in adverse events between groups was dermatitis. Among patients using TTFields, 43% experienced dermatitis, which was mostly grade 1-2. Only 2% of patients in the standard-of-care arm experienced dermatitis. There were no deaths attributable to TTFields therapy and there were no differences in rates of pneumonitis or other immune-related adverse events. Additionally, she said there were no notable differences in health-related quality of life when TTFields was added to standard of care.
“The LUNAR study met its primary endpoint of overall survival,” Dr. Leal concluded. “TTFields therapy with standard of care provided a statistically significant and clinically meaningful 3-month improvement in median overall survival versus standard of care alone with no added systemic toxicities. A statistically significant 8-month increase in median overall survival was demonstrated with TTFields therapy and an ICI.”
She said TTFields therapy is a potentially paradigm shifting new treatment modality and should be considered a part of standard care for mNSCLC following progression after platinum-based chemotherapy.
However, as discussant Rebecca Suk Heist, MD, MPH, Assistant Physician, Massachusetts General Hospital, Boston, pointed out, there are unanswered questions.
“The major caveat for me is that the study design does not reflect the current standard of care,” Dr. Heist said. “The LUNAR study met its primary endpoint. However, the standard of care used in this study has become outdated and is not our current standard. The lack of clarity on the mechanism of action also gives me pause.”
That said, as a clinician Dr. Heist said she always welcomes new modalities to treat lung cancer.
“Tumor treating fields are noninvasive, however, they are bulky,” she said. “I’d be interested to see what the quality-of-life outcomes are.”
Additional studies evaluating TTFields therapy with current standard of care for first-line metastatic and locally advanced NSCLC are in progress, Dr. Leal said.