
Bruce E. Johnson, MD

Mizuki Nishino, MD, MPH
Precision therapy using molecular targeting agents has become mainstream treatment for patients with advanced NSCLC harboring oncogenic driver mutations, as heralded by EGFR TKIs for EGFR-mutant NSCLC.1,2,3,4
Most patients with EGFR-mutant advanced NSCLC treated with an EGFR TKI have an initial response, often with remarkable tumor shrinkage, and then achieve their tumor nadir (the smallest tumor burden since the initiation of therapy) usually between 6 and 12 months of therapy. After reaching the nadir, however, their tumors will often stabilize in volume, then slowly grow back while the patient is on therapy; eventually disease progression ensues.
The original EGFR TKI is often continued beyond the initial identification of disease progression, following Response Evaluation Criteria in Solid Tumors (RECIST), because the tumors tend to grow slowly over time, for many months to a few years, without worsening symptoms or clinical deterioration.5,6,7 Currently, there are no objective criteria to guide precision therapy beyond RECIST progression for these patients. The decision whether to continue initial therapy or to switch to alternative therapies are generally made subjectively based on “continued clinical benefit,” as judged by treating clinicians.
Serial CT imaging objectively characterizes tumor volume dynamics and provides quantitative metrics such as initial tumor shrinkage and tumor growth rate after nadir. These metrics have the potential to serve as predictive markers for clinical outcome and can inform treatment decisions.8,9,10 However, the concept of tumor growth rate over time is not incorporated in RECIST.
Rather, RECIST defines tumor progression as occurring when patients experience a 20% or greater increase in tumor size of “indicator” lesions post nadir, independent of the length of time from the nadir. Patients whose tumors show a 20% or greater increase in 2 months from the nadir compared to those exhibiting a 20% or greater increase over 12 months may warrant different management.
From the Editor…
“Tumor growth rate kinetics are an interesting and potentially useful tool in therapeutic decision-making, particularly in patients whose tumors harbor oncogenic drivers; but this metric cannot be instituted in a vacuum. Patients with tumor growth rates of ≤0.12/month who are increasingly symptomatic from their cancers still warrant alternative therapeutic strategies.”
—Corey J. Langer
Editor, ILCN
To address this issue, investigators analyzed serial CT imaging to define the volumetric tumor growth rate post nadir in patients with EGFR-mutant advanced NSCLC treated with the EGFR TKIs erlotinib or gefitinib. They determined a reference value of 0.12/month for the logarithm of the tumor volume (logeV, originally measured in mm3) as a cut point for tumor growth rate, which was reproduced in an external validation cohort.9,10
The results indicated that a tumor growth rate of < 0.12/month for logeV can define slow tumor growth for patients with EGFR-mutant disease who are receiving EGFR TKI therapy and may be used to help guide treatment decisions beyond RECIST progression. Building on these observations, the authors have recently investigated the association between tumor growth rate and survival in patients with EGFR-mutant advanced NSCLC,11 to further support the use of tumor growth rate in therapeutic decision-making.
In the study published in JCO Precision Oncology,11 71 patients with EGFR-mutant advanced NSCLC treated with erlotinib underwent study of CT tumor volume dynamics during therapy (see Fig. 1).12,13 The reference value of 0.12/month for logeV was used to define slow versus fast growth after nadir. The OS of 48 patients with slow tumor growth was significantly longer compared to 23 patients with fast tumor growth.11 Other variables including age, sex, race, EGFR mutation type, prior chemotherapy, and the number and types of subsequent therapies had no significant impact on survival time.11
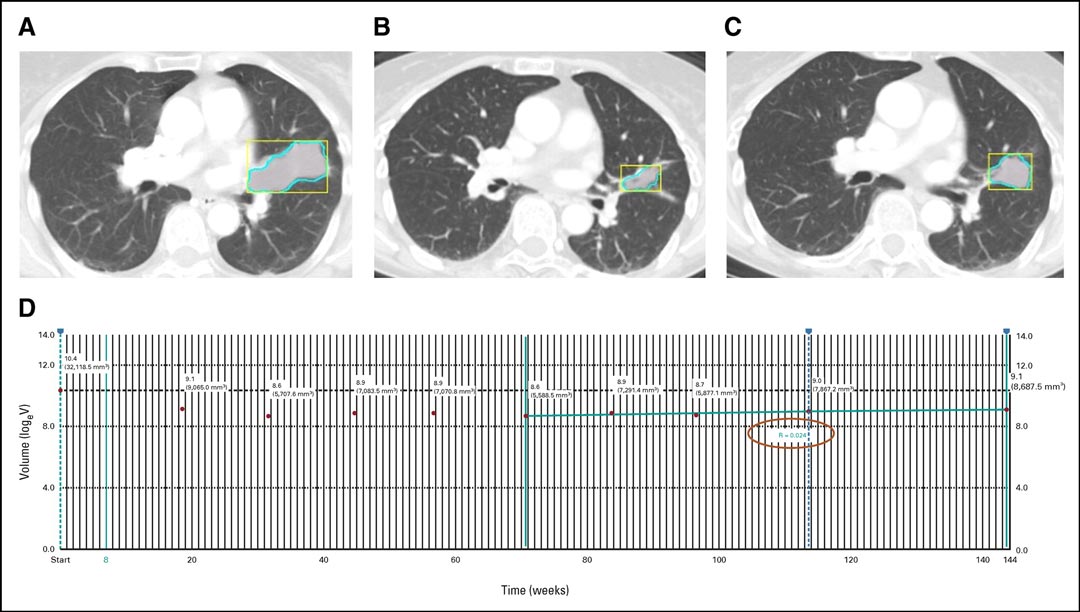
Fig. 1. Tumor Volume Tracking and Tumor Growth Calculation by the Analytic Module.
A 63-year-old woman with EGFR-mutant adenocarcinoma of the lung treated with erlotinib, with the left upper lobe mass at baseline (A). The tumor volume decreased during therapy and reached the nadir at 16.3 months (B). After nadir, the tumor volume gradually increased over 1.5 years (C). The trend graph for logeV is displayed by the module on the Vitrea workstation, and the tumor growth rate for logeV calculated by the module was 0.024/month (D).
Reprinted with permission from the American Society of Clinical Oncology and Wolters Kluwer Health. Nishino M, Lu J, Hino T, et al. Tumor Growth Rate After Nadir Is Associated With Survival in Patients With EGFR-Mutant Non–Small-Cell Lung Cancer Treated With Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. JCO Precision Oncology. 2021;5:1603-1610. https://ascopubs.org/doi/10.1200/PO.21.00172
The study showed, for the first time, that slower tumor growth rate after nadir is associated with longer survival in patients treated with EGFR TKIs and provided a scientific basis for using tumor growth rate as a guide for treatment decision making in the “precision therapy” of advanced NSCLC. Tumor growth rate can be obtained using chest CT scans that are routinely obtained as a part of clinical follow-up of patients with advanced NSCLC during therapy. These scans are usually performed every 6 to 8 weeks, without any additional testing or interventions. The threshold of 0.12/month to differentiate slow versus fast tumor growth can be useful for clinicians as an objective supplement to the existing, subjective, and less precise guidance in which treatment decision-making with EGFR TKIs made.
The results of the study indicated that patients with a tumor growth rate of 0.12/month or less may safely remain on EGFR TKIs in the absence of new symptoms, whereas alternative or additional therapy may need to be considered for those with a tumor growth rate greater than 0.12/month.
The planned next steps of this investigation include validation of the results in prospective cohorts of patients treated with EGFR TKIs, including the newer and more effective osimertinib, and the application of the tumor growth rate kinetics to patients with other genomic drivers, including patients with ALK-positive disease currently being treated with ALK inhibitors.14,15
In addition, the authors are actively working to further reduce technical barriers, to translate the approach more widely into the clinical practice setting by developing and optimizing an automated analytic module that can provide graphical display of tumor volume dynamics with an instant calculation of tumor growth rate from serial chest CT scans.13 The research presented in this study illustrates how radiologists and imaging can contribute to further advances to support clinical decision making by addressing an important question that has plagued oncologists.
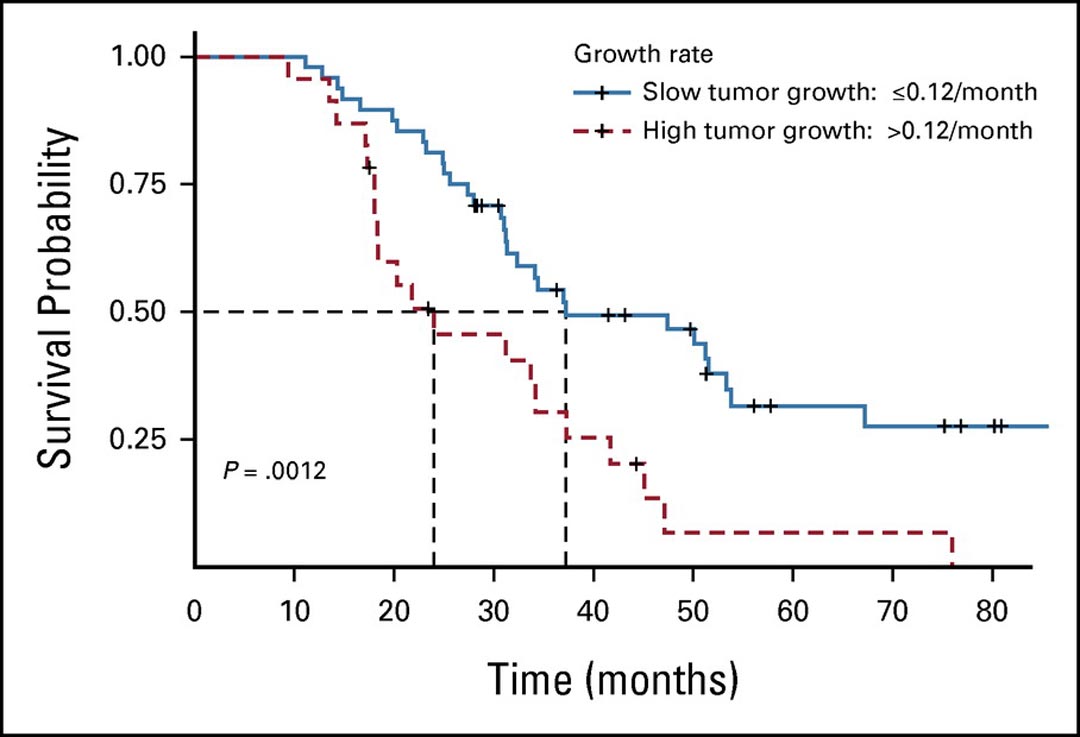
Fig. 2. Kaplan-Meier Estimates of OS of Patients, Dichotomized at 0.12/Month for logeV for Slow vs. Fast Growth.
Forty-eight patients with a tumor growth rate of 0.12/month or less (slow growth) had significantly longer survival compared with 23 patients with a tumor growth rate greater than 0.12/month (fast growth; median OS: 37.8 vs. 25.0 months, respectively, log-rank p = 0.0012).
Reprinted with permission from the American Society of Clinical Oncology and Wolters Kluwer Health. Nishino M, Lu J, Hino T, et al. Tumor Growth Rate After Nadir Is Associated With Survival in Patients With EGFR-Mutant Non–Small-Cell Lung Cancer Treated With Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. JCO Precision Oncology. 2021;5:1603-1610. https://ascopubs.org/doi/10.1200/PO.21.00172
References:
- 1. Johnson BE. Divide and Conquer to Treat Lung Cancer. N Engl J Med. 2016;375:1892-1893.
- 2. Nishino M, Hatabu H, Johnson BE, McLoud TC. State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology. 2014;271:6-27.
- 3. Park H, Sholl LM, Hatabu H, Awad MM, Nishino M. Imaging of Precision Therapy for Lung Cancer: Current State of the Art. Radiology. 2019;293:15-29.
- 4. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-1500.
- 5. Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150-5155.
- 6. Mok TS. Living with imperfection. J Clin Oncol. 2010;28:191-192.
- 7. Park K, Yu CJ, Kim SW, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol. 2016;2:305-312.
- 8. Nishino M, Dahlberg SE, Cardarella S, et al. Tumor volume decrease at 8 weeks is associated with longer survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR TKI. J Thorac Oncol. 2013;8:1059-1068.
- 9. Nishino M, Dahlberg SE, Cardarella S, et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy: developing criteria to continue therapy beyond RECIST progression. Cancer. 2013;119:3761-3768.
- 10. Nishino M, Dahlberg SE, Fulton LE, et al. Volumetric Tumor Response and Progression in EGFR-mutant NSCLC Patients Treated with Erlotinib or Gefitinib. Acad Radiol. 2016;23:329-336.
- 11. Nishino M, Lu J, Hino T, et al. Tumor Growth Rate After Nadir Is Associated With Survival in Patients With EGFR-Mutant Non–Small-Cell Lung Cancer Treated With Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. JCO Precision Oncology. 2021:1603-1610.
- 12. Nishino M, Guo M, Jackman DM, et al. CT tumor volume measurement in advanced non-small-cell lung cancer: Performance characteristics of an emerging clinical tool. Acad Radiol. 2011;18:54-62.
- 13. Nishino M, Wakai S, Hida T, et al. Automated image analysis tool for tumor volume growth rate to guide precision cancer therapy: EGFR-mutant non-small-cell lung cancer as a paradigm. Eur J Radiol. 2018;109:68-76.
- 14. Nishino M, Hida T, Kravets S, et al. Tumor volume dynamics and tumor growth rate in ALK-rearranged advanced non-small-cell lung cancer treated with crizotinib. Eur J Radiol Open. 2020;7:100210.
- 15. Hida T, Dahlberg SE, Lydon CA, et al. Tumor Volume Analysis as a Predictive Marker for Prolonged Survival in Anaplastic Lymphoma Kinase-rearranged Advanced Non-Small Cell Lung Cancer Patients Treated With Crizotinib. J Thorac Imaging. 2020;35(2):101-107.





