Monday morning’s Presidential Symposium featured the four abstracts judged the best for science of those submitted for presentation at WCLC 2022. The abstracts reflected the broad, multidisciplinary appeal of the meeting—ranging from a smoking cessation study to a practice-changing study comparing two surgical approaches for non-small cell lung cancer (NSCLC) patients with small tumors, to updates from two trials of immune-targeted therapies.
“These were the abstracts we felt were the best of the best,” said IASLC President Heather A. Wakelee, MD, FASCO, chief of the division of oncology at Stanford University and deputy director of the Stanford Cancer Institute. “We also were really excited to realize, as we were looking at the top abstracts, that we were going to be able to present from a multidisciplinary approach and examine a lot of key topics that are really critical as we work to conquer thoracic malignancies worldwide.”

Nasser Khaled Altorki, MD, presented results from a large international study (abstract 3051) of patients with NSCLC tumors ≤2 cm that demonstrated that sublobar surgery was noninferior to lobectomy, which has been the surgical standard of care for cT1aN0 NSCLC since 1995. The results of the trial, combined with results from the JCOG 0802 trial released earlier this year, establish sublobar resection as the standard of care for patients with peripheral cT1aN0 NSCLC (≤2 cm) without metastases to major hilar and mediastinal lymph nodes, Dr. Altorki said.
Dr. Altorki and his research colleagues conducted CALGB140503 (Alliance), a multicenter noninferiority phase III trial in which NSCLC patients clinically staged as T1aN0 with tumor measuring ≤2 cm were randomly assigned to lobar or sublobar resection. The primary endpoint was disease-free survival (DFS) and secondary endpoints included overall survival (OS) and the difference in pulmonary functions at 6 months postoperatively between arms.
The trial enrolled 1,080 patients with clinical stage 1A NSCLC between June 2007 and March 2017. To be eligible for the study, patients had to have pathologically confirmed NSCLC and node-negative disease at level 10, and up to two mediastinal stations prior to randomization. Of the 1,080 patients enrolled, 697 patients were intraoperatively randomly assigned to either lobar (357 patients) or sublobar (340 patients) resection. Basic demographic and clinical characteristics were balanced between the two arms of the trial and minimally invasive approaches such as video-assisted thoracoscopic surgery were used for 80% of all resections.
The research team followed the patients for seven years and determined the noninferiority significance boundary had not been crossed. Thirty- and 90-day mortality were 1.1% and 1.7% after lobar resection and 0.6% and 1.2% after sublobar resection.
“If you combine the results of this trial with the results of the Japanese trial (JCOG 0802), I think we have pretty strong evidence that in this cohort of highly selected patients—and it’s important to point out that these are patients who met strict eligibility criteria before surgery and during surgery—that it’s safe, advisable, and in fact the new standard of care to do a sublobar resection,” said Dr. Altorki, professor of cardiothoracic surgery and director of the division of thoracic surgery at New York Presbyterian-Weill Cornell Medical Center.
Progression Free Survival and Overall Survival in the NADIM II Study

Mariano Provencio, MD, PhD, scientific director of the Health Research Institute of the Puerta de Hierro University Hospital and professor of medicine at the Autonomous University of Madrid, Spain, presented new data (abstract 1988) from the NADIM II trial, a follow-up of the NADIM trial in which neoadjuvant chemoimmunotherapy was shown to be highly effective in patients with resectable stage IIIA NSCLC.
NADIM II (NCT03838159) was an open-label, randomized, two-arm, phase II, multicenter clinical trial sponsored by the Spanish Lung Cancer Group (GECP). Patients with resectable clinical stage IIIA NSCLC, ECOG PS 0-1, and no known EGFR/ALK alterations were randomized to receive nivolumab 360mg + paclitaxel 200mg/m2 + carboplatin for three cycles every 21 days (+/- 3 days) as neoadjuvant treatment followed by surgery, or chemotherapy alone followed by surgery.
Patients with R0 resection, confirmed by pathological evaluation, initiated adjuvant administration of nivolumab (480 mg Q4W) within the third to eighth week from surgery and continued this agent for six months. The primary endpoint was pathological complete response (PCR); progression-free survival (PFS), OS, and biomarker analysis were secondary endpoints of the trial.
Median follow-up time was 21.9 months. At the time of data cutoff (March 2021), PFS at 24 months was 67.3% for patients treated with nivolumab plus chemotherapy versus 52.6% for patients treated with chemotherapy.
Overall survival at 24 months was 85.3% with nivolumab plus chemotherapy versus 64.8% with chemotherapy. In the experimental arm, programmed death-ligand 1 (PD-L1) expression (≥1%) identified patients with significant improvement in PFS. The PCR rate was 36.2% in the experimental arm versus 6.8% in the control arm. None of the patients exhibiting a PCR has had disease progression or died.
“NADIM II demonstrates that the efficacy of nivolumab in combination with platinum-based chemotherapy in patients with resectable stage IIIA NSCLC is supported by survival data,” Dr. Provencio said. “The benefit is especially pronounced in patients whose tumors express PD-L1 and in those patients achieving PCR.”

IMpower010: Overall Survival Interim Analysis of a Phase III Study of Atezolizumab vs. Best Supportive Care in Resected NSCLC
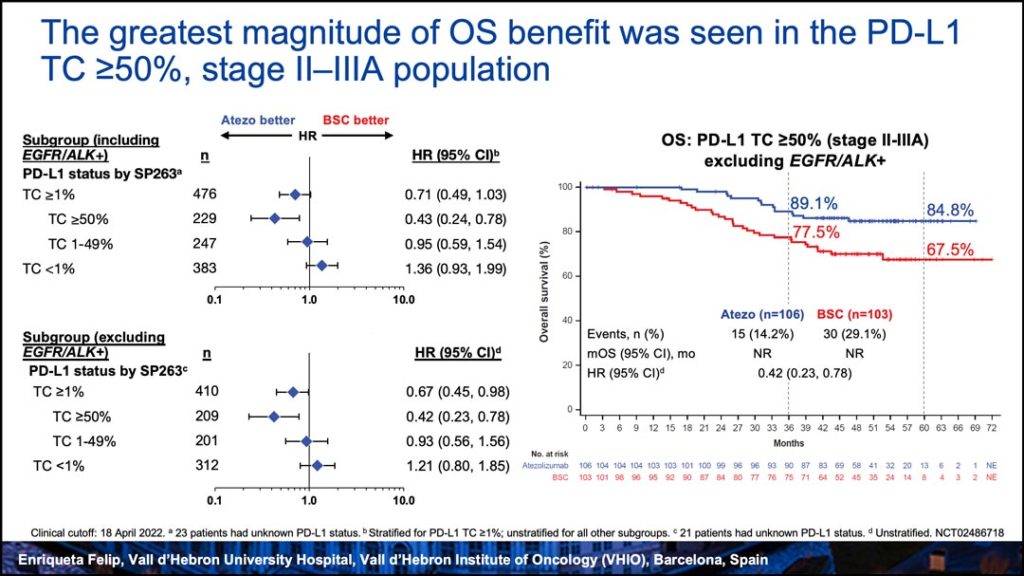
An interim analysis (abstract 2332) of OS data from the IMpower010 trial showed an OS trend in favor of atezolizumab in the PD-L1 tumor cell (TC)>1% stage II-IIIA population, but not in the all-randomized stage II-IIIA or intent-to-treat (ITT) population, which included patients with stage IB, according to Enriqueta Felip, PhD, head of the Lung Cancer Unit, Oncology Department, at Vall d’Hebron University Hospital, Barcelona, Spain.
The highest magnitude of OS improvement was observed in patients with stage II-IIIA disease whose tumors expressed PD-L1 TC≥50% (HR=0.43, 95% CI: 0.24–0.78), Dr. Felip reported.
IMpower010 previously yielded a statistically significant DFS benefit with adjuvant atezolizumab compared with best supportive care (BSC) in patients with resected NSCLC following platinum-based chemotherapy. Based on these findings, atezolizumab was approved as adjuvant treatment after complete resection and platinum-based chemotherapy in patients with PD-L1 TC≥1% stage II-IIIA NSCLC in the U.S., China, and other countries; and in PD-L1 TC≥50% stage II-IIIA NSCLC in the European Union (excluding EGFR/ALK+) and elsewhere. countries.
The key secondary OS endpoint was not mature at the time of the previous IMpower010 DFS interim analysis. The data presented Monday evaluated OS and safety with 13 months of additional follow-up.
“This OS analysis shows a promising trend in favor of atezolizumab over BSC in the PD-L1 TC≥1% stage II-IIIA population and a clinically meaningful improvement in the PD-L1 TC≥50% stage II-IIIA population, with OS improvements observed across most subgroups. No separation was observed for the ITT population or the all-randomized, stage II-IIIA populations. We will continue to follow patients in this study as data mature,” said Dr. Felip, adding that IMpower010 will continue to the final DFS analysis with further OS analyses.

Personalized Smoking Cessation Support in a Lung Cancer Screening Program: The Yorkshire Enhanced Stop Smoking Study (YESS)
Lung cancer patients who attended a lung cancer screening (LCS) event and then participated in a personalized smoking cessation study achieved smoking abstinence rates of more than 30% in the Yorkshire Enhanced Stop Smoking (YESS) trial, according to research (abstract 1863) presented by Rachael L. Murray, PhD, professor of population health at the University of Nottingham, United Kingdom.
To test the effectiveness of a personalized approach to smoking cessation, United Kingdom-based researchers developed the YESS trial, which offered support on an opt-out basis to all eligible smokers attending a lung health check (LHC) event, which included low-dose CT screening. The double-blind, randomized controlled trial compared an enhanced, personalized smoking cessation support program with scripted behavioral support delivered by a trained smoking cessation practitioner vs. the same support without the personalized support. The personalized support included a booklet containing CT images of the participants’ own heart and lungs, annotated where appropriate to highlight emphysema or coronary artery calcification with accompanying explanatory text. The randomization occurred after one month of cessation support for all patients.
The primary outcome in the trial was a point prevalent (self-reported) carbon monoxide validated test of smoking cessation three months after the LHC event and two months after randomization in the YESS trial. Secondary outcomes included carbon monoxide-validated cessation at four weeks and 12 months, and self-reported cessation at four weeks, three months, and 12 months following the event.
Validated seven-day abstinence rates in the trial were 33.6% in the intervention group, 30% in the control group (unadjusted OR 1.17, 95% CI, 0.90–1.54) at three months post-LHC and 29.2% and 28.6% (unadjusted OR 1.03, 95% CI 0.78–1.36) at 12 months post-LHC. Subgroup analyses indicated a significant gender interaction at three and 12 months (p=0.002 and p=0.001, respectively), with the intervention more effective in female trial participants. There was no significant effect of booklet content (presence/absence of emphysema or CAC) on quit rates.
“The presence of a co-located stop smoking service and offer of immediate, opt-out delivery of behavioral and pharmacological support for quitting results in high uptake by people who smoke and attended a lung screening event,” Dr. Murray said. “Quit rates were considerably higher three months after the lung health check regardless of adding the personalized intervention, reinforcing the need for continued support.”

Cigarette Labeling Differs Greatly by Region
When it comes to cigarette packaging in Austria and elsewhere in the European Union, there is no lack of graphic imagery in product warnings. Packaging includes provocative images of diseased lungs, tracheotomy patients and amputees (presumably from the effects of tobacco-induced peripheral vascular disease). Such graphic imagery has not existed traditionally in the US, where product warnings are watered down, text only. It begs the question, do visual warnings improve the success rates of smoking cessation or prevention events?
— Corey Langer, MD, and Erin Jungmeyer. Photo courtesy of Kathleen Gambino.





