A broad range of ongoing research seeks to identify effective targeted therapies for patients with MET-dysregulated non-small cell lung cancer (NSCLC). MET dysregulation in NSCLC may result from MET exon 14 (METex14) skipping mutations, MET amplification, or MET overexpression, said Noemi Reguart, MD, PhD, of the Hospital Clinic, Barcelona, Spain, during her presentation at the 2022 Asia Conference on Lung Cancer (ACLC).
“When we’re talking about MET-targeting therapies, we have to think about the biomarker underlying the disease,” she said. “We have METex14 skipping mutation, which is present in 3% to 4% of patients. We also have other MET alterations, including amplification, which we see in 5% of NSCLC patients and increases to 17% of patients in the setting of EGFR resistance. Then we have overexpression, which is found in 24% of NSCLC cases and up to 50% of cases with EGFR resistance.”
During her ACLC lecture, Dr. Reguart reviewed the research that can inform treatment decisions for patients with MET-dysregulated NSCLC.
“The landscape of MET-targeted therapies is expanding and new therapies, including tyrosine kinase inhibitors, monoclonal antibodies, and antibody drug conjugates are showing promising activity in MET-dysregulated non-small cell lung cancer,” she said.
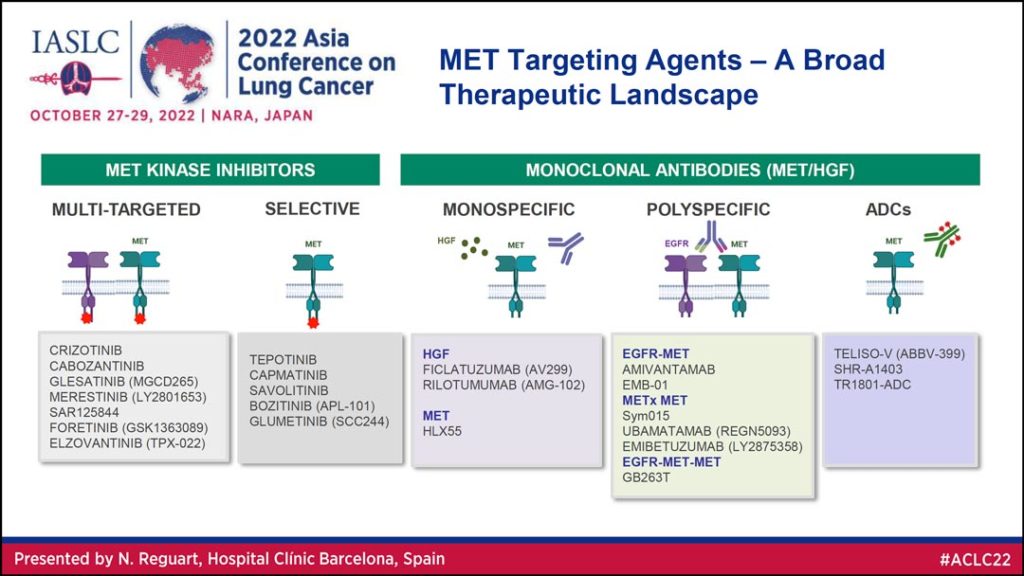
Dr. Reguart began with a high-level overview of MET targeting agents, which she said can be divided into two primary categories: Kinase inhibitors and monoclonal antibodies (Table 1). Under these broader umbrellas, kinase inhibitors can be multi-targeted or selective and monoclonal antibodies can be monospecific, polyspecific, or antibody drug conjugates.
Tyrosine Kinase Inhibitors
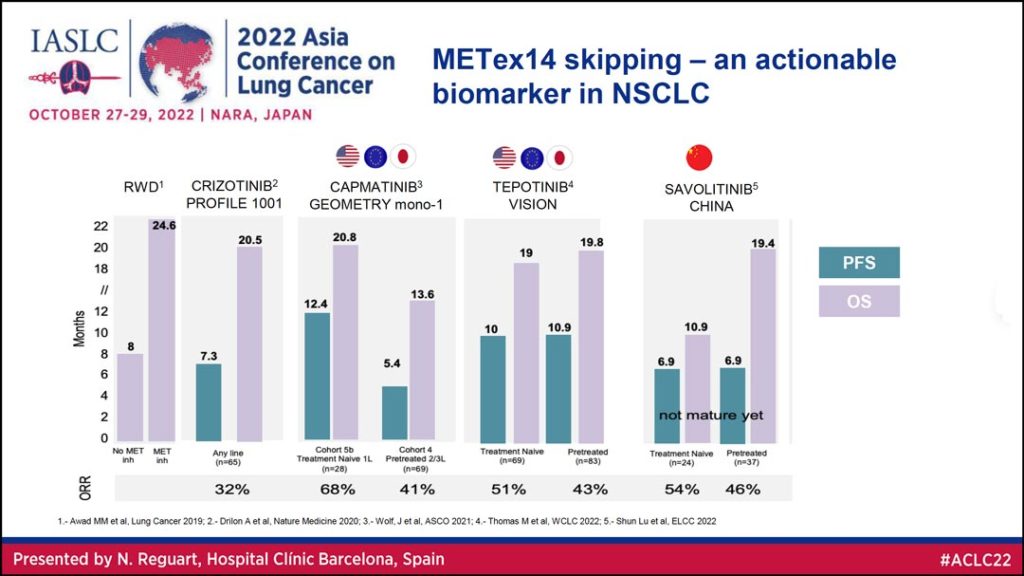
Looking at the data from studies of patients with METex14 skipping mutations shows this is an actionable biomarker in NSCLC, Dr. Reguart said. She reviewed data (Table 2) on overall survival (OS) and progression free survival (PFS) for MET tyrosine kinase inhibitors (TKIs), including crizotinib, capmatinib, tepotinib, and savolitinib. The overall response rate (ORR) for these agents ranged from 32% to almost 70% in patients with METex14 skipping mutations.
For NSCLC patients with METex14 skipping mutations, capmatinib and tepotinib are now the standard of care in the US, Europe, and Japan; and savolitinib is the standard of care in China.
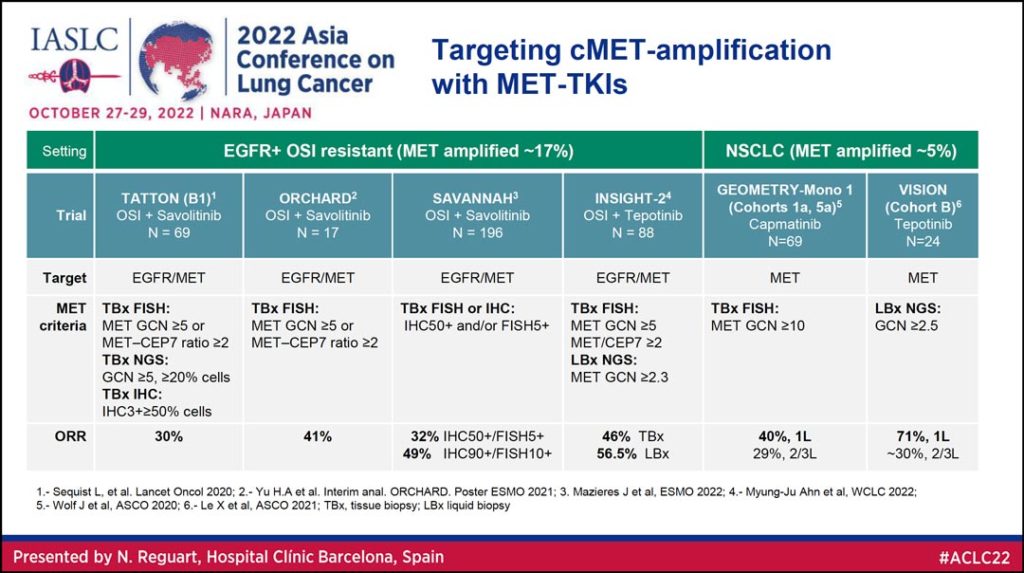
MET TKIs are also being explored as potential therapy for targeting MET amplification (Table 3).
“We have data coming from two settings of the disease,” Dr. Reguart said. “First in those patients with EGFR+ mutations in the resistance setting in which the combination of osimertinib and savolitinib or osimertinib and tepotinib can achieve really nice antitumor activity with an overall response rate between 30% and 40%. Second, you can see very nicely the activity of capmatinib and also tepotinib based on the level of MET amplification with responses of 40% up to 71% in those with high MET amplification.”
Monoclonal Antibodies
Dr. Reguart said monoclonal antibody (mAB) research in MET-dysregulated NSCLC includes the phase I CHRYSALIS trial, which demonstrates antitumor activity of amivantamab monotherapy with overall response rates of 57% in treatment naïve patients (n=9), 47% in patients with no prior MET-targeted therapy (n=18), and 17% in patients who had previous MET-targeted therapy (n=28).
She also reviewed data on MET-antibody drug conjugates (ADCs).
“ADCs use a different modality that combines antibodies with a targeted cytotoxic payload,” Dr. Reguart said. “And there are different types of them depending on the antibody and the type of chemotherapy linked to the antibody.
“When we’re talking about ADCs, it’s important to think about the biomarker, because here what we want is not to see oncogenicity but to have expression in the membrane in order for the antibody to bind and deliver the cytotoxic payload.”
One of the MET-ADCs most advanced in testing is telisotuzumab vedotin, which is in phase 3 development. A phase 2 study of telisotuzumab vedotin monotherapy in MET+ NSCLC showed an ORR of 36.5% in patients with IHC 3+ in >25% of cells and an ORR of 52% in patients with IHC 3+ in >50% of cells. A phase 1/1b study of telisotuzumab vedotin plus osimertinib in patients with MET+ EGFR resistant NSCLC yielded an ORR of 58%.
Dr. Reguart concluded by stressing the importance of biomarker selection and standardized testing assays to achieve optimal results for MET-dysregulated NSCLC patients as their treatment options expand.