Data presented during the Best Fellows Oral Abstract Session at the 2023 Targeted Therapies of Lung Cancer meeting demonstrated first-line osimertinib can be effective in select patients with uncommon EGFR mutations. However, presenter and second-year medical student Tia Cheunkarndee, BA, said the heterogeneity of mutations and response rates highlight the need for further study. Registered TTLC 23 attendees can watch the full session on-demand.
Cheunkarndee, of Johns Hopkins University School of Medicine and the Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland, presented data from a study of first-line osimertinib in patients with uncommon EGFR-mutated non-small cell lung cancer (NSCLC). She said an estimated 10 to 20% of EGFR mutations are uncommon.
“These rare mutations encompass a wide variety of mutations across the tyrosine kinase domain spanning exons 18 through 21,” Cheunkarndee said. “The low frequency of each of these individual mutations makes them challenging to study.”
Current Landscape
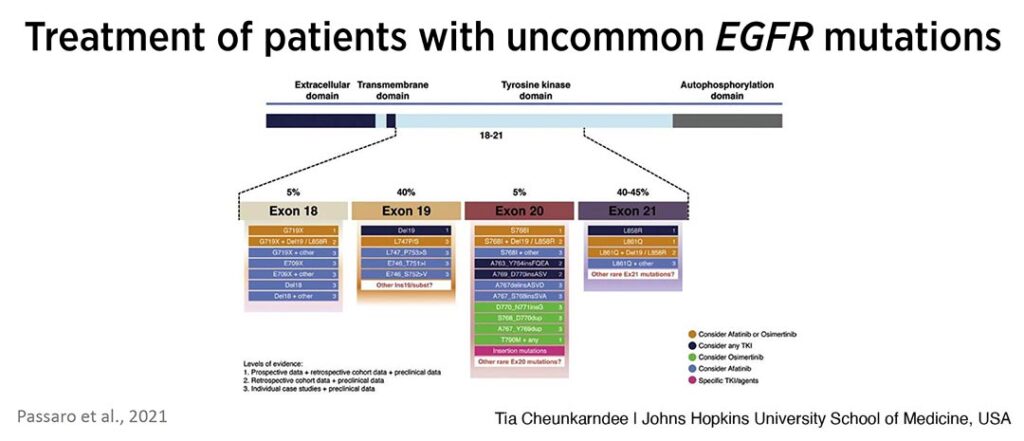
Cheunkarndee shared data from a 2021 review by Passaro et al (see Fig. 1) that outlines treatment recommendations by mutation and provides the levels of evidence for each. She reminded the audience that the only US Food and Drug Administration approved treatment for uncommon EGFR mutations is afatinib and it is only approved for a few mutations—G719X, S768I, and L861Q.
“For the remainder of the uncommon mutations shown here, most have at most case study or retrospective levels of evidence regarding which TKI to use,” Cheunkarndee said. “Many patients are currently treated with afatinib as osimertinib is newer and more regarding its use is unknown.”
Much of what is known about osimertinib in the first-line comes from the FLAURA study, which looked at first-line osimertinib for NSCLC harboring common EGFR mutations. The FLAURA study showed an overall response rate of 80%, median progression free survival (PFS) of 19 months, a median overall survival (OS) of 39 months, and a median exposure to first-line osimertinib of 21 months.
“However, the response of EGFR mutated non-small cell lung cancer harboring uncommon mutations to osimertinib still remains relatively unclear,” Cheunkarndee said. “Studies looking at first-line osimertinib in patients with uncommon mutations have shown response rates and duration of response that are lower than what is seen among patients harboring common mutations receiving first-line osimertinib.
“It’s important to remain cognizant of the vast heterogeneity that exists among these uncommon EGFR mutations and how this can translate into a lot of heterogeneity among studies on this topic as well.”
Looking Deeper at Uncommon Mutations
For the Hopkins study, the investigators identified patients who were initiated on first-line osimertinib for stage III/IV EGFR mutated NSCLC at Johns Hopkins from November 2016 through August 2021. Cheunkarndee said the team identified 144 patients who fit into this category, and of those they identified 26 patients who harbored uncommon EGFR mutations.
“For how we classified uncommon EGFR mutations, we essentially included any mutation that was not an exon 20 insertion because osimertinib is not indicated for these patients,” Cheunkarndee said. “Furthermore, we also included any exon 19 deletion that was not the more common E746_A750 deletion… Finally, we also included any compound mutation as long as it included an uncommon mutation that was within the kinase domain.”
Of the cohort of 26 patients, 17 harbored single mutations and nine had compound mutations. Nearly half the cohort and a large majority of the single mutation group was also comprised of rare exon 18 mutations.
“While these may have been included in larger prospective studies along with the classic exon 19 mutations, we ultimately decided to include them because we have seen that these rare mutations may have less well defined and less optimal responses to osimertinib than the more common E746_A750, which encompasses more than 80% of exon 19 mutations.”
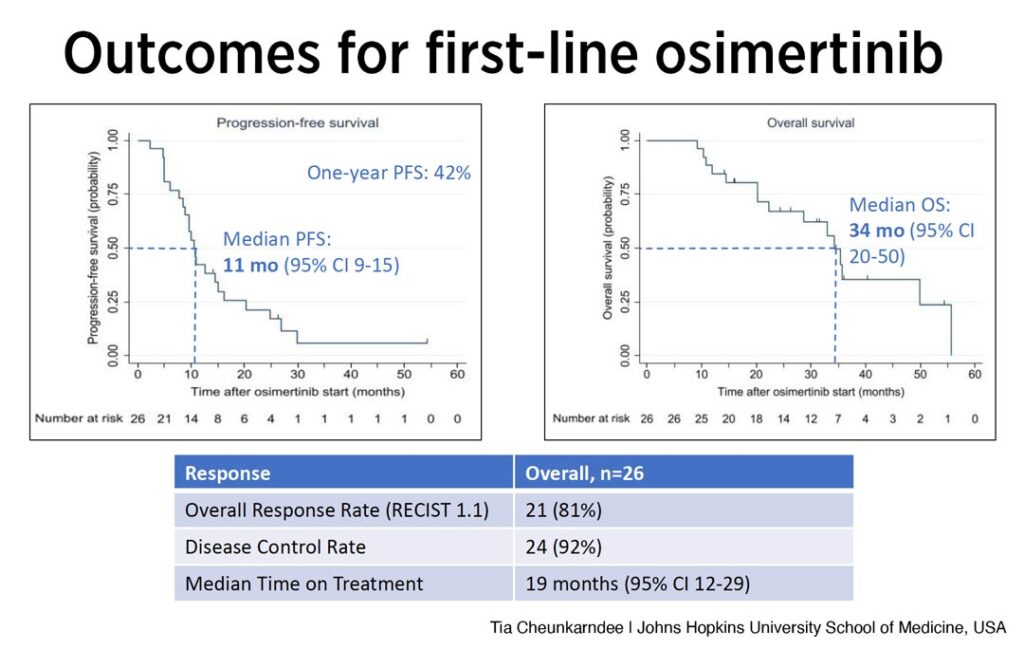
Looking at the results (see Fig. 2), PFS of 11 months was lower than what was seen among patients with classical mutations in FLAURA (19 months).
Additionally, Cheunkarndee said 42% of patients remained progression free at one year. The median overall survival was 34 months and the overall response rate as per RECIST criteria was relatively robust with 81% of patients achieving a partial response. The disease control rate was 92% and the median time on first-line osimertinib was 19 months.
Cheunkarndee then reviewed the results in more detail for the single mutation and compound mutation groups (see Fig. 3).
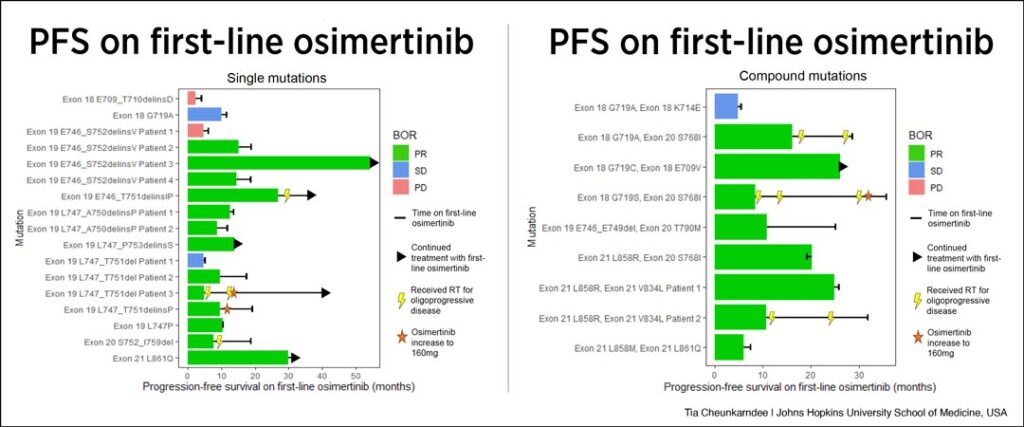
For the cohort of patients with single mutations, Cheunkarndee said that while they observed a lack of durable response for the two exon 18 mutations at the top of the graph, the response rate was generally favorable for the mutations with relatively undefined responses to first-line osimertinib. However, both response and persistence of response are very heterogeneous, she said.
“For instance, among this group of patients who all have various exon and T mutations, we can see a wide range of responses with some patients receiving no benefit and some remaining on therapy for years,” she said. “And even more specifically four patients had the same exon 19 mutation—the E746_S752delinsV. Of these four patients, one experienced progressive disease as their best response while another remains progression free and still on first-line osimertinib at 54 months.”
Looking at compound mutations, the study showed similar results with generally favorable responses overall, but again, widely heterogeneous durations of response. Cheunkarndee said the team was interested to see some favorable responses among the group of patients with various exon 18 compound mutations since their single mutation counterparts had relatively poor responses to first-line osimertinib.
“Taking a step back though and thinking about the cohort overall, we demonstrated that several patients with uncommon EGFR mutations did receive some benefit from first-line osimertinib, but the time to development of resistance is very variable across the board,” she said. “Additionally, some patients were able to remain on this line of therapy after progression with the addition of local therapy, while others experienced more widespread progression.”