Patients with treatment-naïve resectable non-small cell lung cancer (NSCLC) who received neoadjuvant durvalumab plus chemotherapy followed by adjuvant durvalumab had improved event-free survival (EFS) and pathologic complete response (pCR) compared to those who received neoadjuvant chemotherapy and placebo, according to a primary analysis from the phase 3 AEGEAN trial. Researcher John Heymach, MD, PhD, presented the findings April 16 during the 2023 American Association for Cancer Research Annual Meeting.
“Non-small cell lung cancer remains the leading cause of cancer mortality, and historically, about half of patients who undergo resection experience recurrence,” said Dr. Heymach, who is chair of Thoracic/Head and Neck Medical Oncology at the University of Texas MD Anderson Cancer Center, Houston. “Anything we can do to increase cure rates for these patients would be a tremendous advance.”
Dr. Heymach pointed out that recent phase 3 trials have demonstrated the benefits of neoadjuvant immunotherapy and adjuvant immunotherapy, and consequently, both therapeutic strategies have received regulatory approval.
“Perioperative regimens (such as the regimen in AEGEAN) that combine these two approaches could potentially enhance the benefits by priming antitumor immunity while the tumor and draining lymph nodes are still in place and by having sustained PD(L)1 pathway inhibition after surgical resection, which could help eradicate micro-metastases,” he said.
In AEGEAN, a randomized, double-blind, placebo-controlled trial, 802 patients with treatment-naïve resectable NSCLC, irrespective of PD(L)1 expression, were randomly assigned (1:1) to receive either neoadjuvant durvalumab plus platinum-based chemotherapy or neoadjuvant placebo plus platinum-based chemotherapy every 3 weeks for four cycles. Following surgery, patients continued to receive either durvalumab or placebo every 4 weeks for up to 12 cycles.
After excluding patients whose tumors had EGFR or ALK alterations, 740 patients in the modified intention-to-treat population (mITT) were assessed in the planned interim analysis evaluating the primary endpoint of EFS. The mITT population was also assessed for the final analyses of the additional primary endpoint of pCR.
“Note that when the study started, patients with EGFR and ALK aberrations were permitted to enroll, but in light of studies that emerged that showed PD(L)1 pathway inhibition might not be the optimal therapy for these patients, the protocol was amended and these patients were (subsequently) excluded,” Dr. Heymach said.
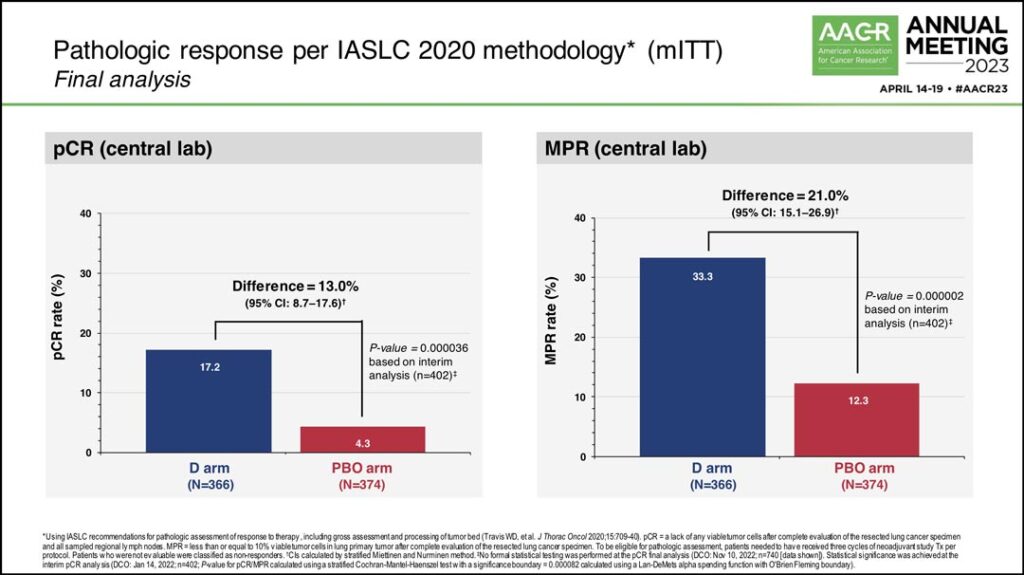
In the final analysis, the pCR rate in the durvalumab arm was 17.2% compared to 4.3% in the placebo arm, a difference of 13%. Dr. Heymach said there was also a statistically significant improvement in major pathologic response (MPR) rate, with 33.3% in the durvalumab arm and 12.3% in the placebo arm. (See Fig. 1) Additionally, 77.6% of patients in the treatment arm and 76.7% of patients in the placebo arm underwent surgery following neoadjuvant therapy.
“Before the study started, there was a concern that giving neoadjuvant immunotherapy might make it harder for some patients to get to surgery,” Dr. Heymach said. “But in fact, we were happy to see an almost identical number of patients get surgery in both arms, suggesting that the inclusion of neoadjuvant durvalumab immunotherapy does not reduce the number of patients who can go on to complete surgery.”
Dr. Heymach also highlighted that slightly higher rates of R0 resections were achieved in the durvalumab arm (94.7%) compared to the placebo arm (91.3%) and 60% of patients went on to adjuvant durvalumab or placebo. Additionally, 23% of patients were still receiving adjuvant therapy at the time of the data lock for the interim analysis.
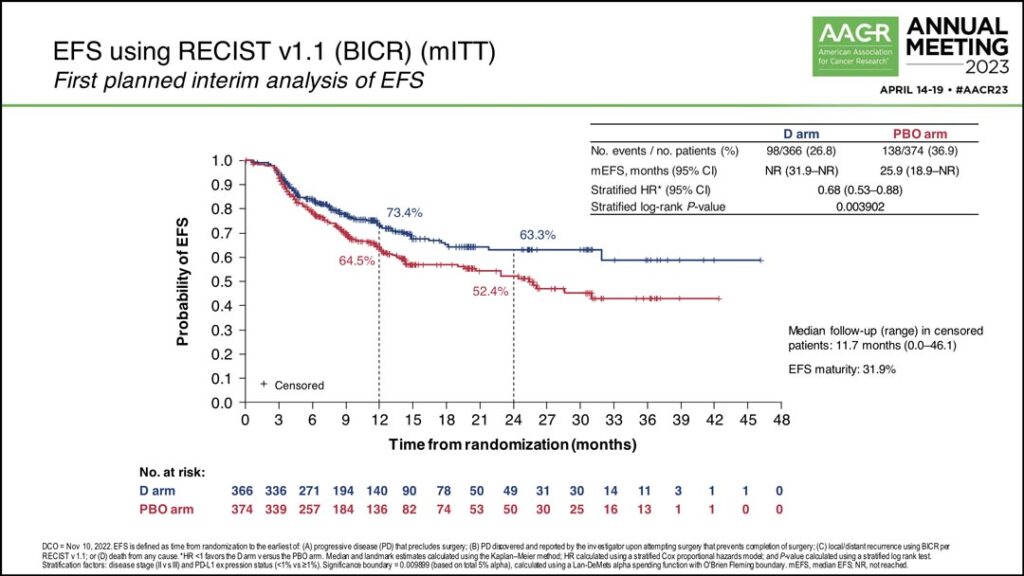
The study also achieved its primary endpoint of improved EFS.
“At the first planned interim analysis the hazard ratio was 0.68 corresponding to a 32% lower risk of an event with a P value of .0039,” Dr. Heymach said. “EFS in the placebo arm was 25.9 months, and EFS was not yet reached in the durvalumab arm. The EFS rate in the durvalumab arm at 24 months was 63%; that was 11% higher than the placebo arm at that landmark.” (See Fig. 2)
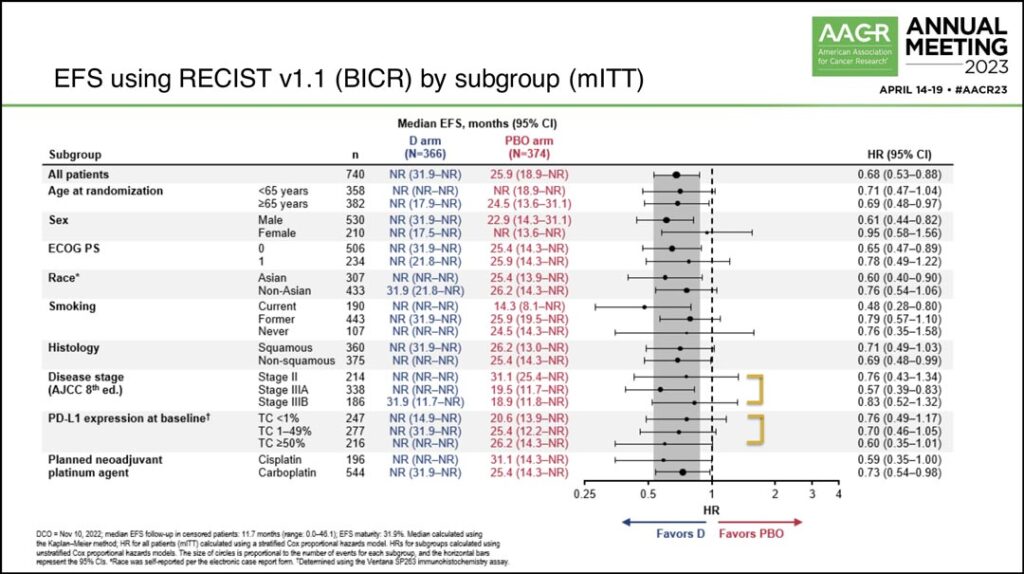
Dr. Heymach also reviewed a forest plot showing the EFS benefit across different subgroups, noting that while patients who were current smokers appeared to have a larger magnitude of benefit, EFS benefit was broadly seen. (See Fig. 3)
“Benefit was seen across disease stages,” he said. “While patients with stage 3A disease had a larger magnitude of benefit, benefit was also seen in stage 2 and 3B disease as well… Benefit was seen regardless of PD(L)1 status. As expected, the benefit was larger in the PD(L)1 greater than 50% subgroup, but benefit was still seen in the PD(L)1 less than 1% subgroup.”
Dr. Heymach concluded his summary of the results by saying he believes the data are incredibly encouraging and that patients with resectable non-small cell lung cancer are likely to have multiple choices moving forward.
“We are excited to see that the trial has achieved both its primary endpoints of improving pCR and significantly reducing the likelihood of disease progression, recurrence, or death,” he said. “The good news for patients with NSCLC is there are now multiple regimens that have shown improvements in outcomes in this setting. This study has laid the foundation that we can build on by designing new combination regimens on top of this effective backbone.”