Since low-dose CT screening was first recommended by the U.S. Preventive Services Task Force (USPSTF) in 2013, debate has continued about how to find the highest proportion of lung cancers at an early stage, when they are still potentially curable.
In March 2021, for the first time since the screening was adopted, the USPSTF updated its eligibility recommendations1 by lowering the pack-year limit and age range of those screened. Nearly doubling the number of people eligible, the new parameters make screening available to those ages 50 to 80 with a smoking history of 20 pack-years or more who currently smoke or have quit within the past 15 years. The criteria also ensure the eligibility of more women and minorities, who previously didn’t qualify due to less extensive smoking histories.
But are those changes substantial enough? Where should the balance fall between attempts to identify lung cancer early and the downsides of screening—from false positives to overtreatment? And, besides increasing screening uptake, what should be done to ensure the identification of as many early lung cancers as possible?
Several research teams recently sought to answer those questions, sharing their findings during the IASLC’s 2021 World Conference on Lung Cancer. One team found advantages to offering CT screening in parallel with the surveillance of patients whose lung nodules had been incidentally detected (Abstract MA10.02). Another considered applying the International Lung Screen Trial (ILST) protocol at first CT screening as a means of planning a surveillance schedule based on malignancy probability (MA10.01). Finally, a third explored the balance between decreasing false positives and delaying diagnosis in lung cancer screening (Abstract MA10.03).
“Since the National Lung Screening Trial results were published 10 years ago, there have been numerous lung screening trials, including the NELSON, that prove that lung cancer screening saves lives. [The question now is:] ‘How do we optimize screening programs?’” said Mary M. Pasquinelli, DNP, APRN, FNP-BC, from the University of Illinois at Chicago and the discussant for the three studies.
“How do programs efficiently and effectively screen individuals and be mindful of healthcare resources while diagnosing lung cancer early to save lives?” she asked. “Optimization needs to be done at the local and institutional levels to fit each individual program and at the national level, as many countries are now beginning lung screening programs or working to optimize their programs.”
Surveillance of Incidentally Detected Nodules Pairs Well with CT Screening
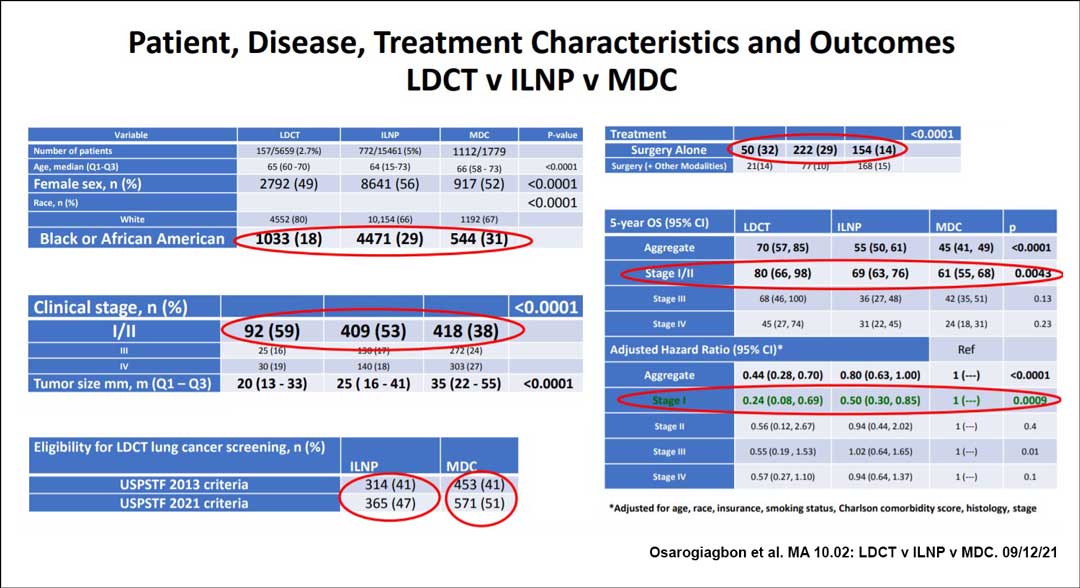
In a prospective study, members of the Thoracic Oncology Research Group at Baptist Cancer Center in Memphis, Tennessee, compared the incidence, treatment modalities, and outcomes associated with early lung cancer based on the strategy used to identify the disease in individuals seen at their large community healthcare system. Cancers were identified either via low-dose CT (LDCT) screening; a concurrently implemented program that provided surveillance of patients with lung nodules that had been detected incidentally through radiologic images; and a multidisciplinary clinic (MDC), used as a comparator.
The incidental lung nodule program (ILNP) detected lung cancer in a higher number and proportion of patients than did the LDCT program, with an added advantage: The majority of individuals with lesions identified through the ILNP would not have qualified for CT screening, even under the 2021 guidelines, presenter Ray U. Osarogiagbon, MBBS, FACP, said.
The researchers also compared overall survival (OS) from date of diagnosis based on the strategy used to detect cancer, finding that LDCT and the ILNP significantly bested results from the MDC.
The team embarked on the study because LDCT and ILNP have “advantages and disadvantages, barriers and facilitators, and it’s not very clear how these two types of programs interact,” Dr. Osarogiagbon said.
Study Details
Between 2015 and 2020, the LDCT program identified 157 patients with lung cancer among 5,659 screened (2.7%); the ILNP identified 772 patients with lung cancer among 15,461 screened (5%); and of the 1,779 patients referred into the MDC, 1,112 were diagnosed with lung cancer. For every case of lung cancer diagnosed via LDCT, five were diagnosed through the ILNP, Dr. Osarogiagbon said.
The study measured a number of parameters associated with lung cancer outcomes. Median tumor size was 20 mm (13-33) in patients diagnosed through LDCT, 25 mm (16-41) in ILNP participants, and 35 mm (22-55) in patients whose disease was identified in the MDC. The team also found that the disease was more frequently stage I or II in patients diagnosed via LDCT (59%) or ILNP (53%) compared with MDC (38%; p ˂ 0.0001). Treatment with surgery alone was most frequent with detection via LDCT (32%), followed by ILNP (29%) and MDC (14%, p ˂ 0.0001).
Five-year OS for those with stage I and II cancers was 80% (66%-98%) with LDCT versus 69% (63%-76%) with ILNP and 61% (55%-68%) with MDC (p ˂ 0.0043). For those with stage I disease, this translated into 76% and 50% reductions in the overall hazard of death, respectively, when diagnosis was made via LDCT or ILNP compared with MDC (p = 0.0009), the team found.
Among the study groups, the researchers found that the population of patients diagnosed via LDCT was the least racially diverse, with 80% of patients being white and 18% Black. The ILNP group was 66% white and 29% Black, and the MDC group was 67% white and 31% Black. Thus, the ILNP is “potentially a means of overcoming racial disparity in early detection,” Dr. Osarogiagbon said.
“Both programs led to earlier-stage diagnoses, more opportunity for surgical resection, less need for adjuvant therapy, and improved survival compared to outside the programs,” he concluded. “Therefore, we feel it is logical to recommend the tandem deployment of both incidental lung nodule and LDCT programs.”
Making Screening More Inclusive
Dr. Pasquinelli said the study’s results confirm that screening works but raise questions about its effectiveness across subgroups of the population.
During the study period, the LDCT clinic at Baptist Cancer Center relied on the USPSTF’s 2013 recommendations, “and we now know that this criteria underselects for African Americans, as they often do not meet the 30 pack-year criteria,” she said. “The updated 2021 criteria may decrease this disparity, but most likely will not eliminate it. Another possibility is the African American cohort may not be using preventative services as much compared to the white cohort, or African American individuals may be more likely to enter the healthcare system via the hospital due to other healthcare issues.”
The fact that the ILNP identified cancers in many who were not eligible for LDCT “points to the importance of continuing to provide research regarding high-risk populations that do not meet the [USPSTF] criteria in order to optimize lung screening eligibility criteria in the future,” Dr. Pasquinelli said.
Tracking Lung Nodules Based on Malignancy Probability
At every LDCT after baseline screening, the management of lung nodules becomes more efficient, due to the ability to track the growth of existing lesions or the appearance of new ones. But initial screens are associated with a higher rate of early recall imaging studies and diagnostic referrals.
A team of researchers from Vancouver General Hospital explored whether the ILST protocol can be applied for a personalized approach to lung nodule management based on malignancy probability; their trial relied on the multivariable PanCan Nodule Malignancy Prediction Model, also known as the Brock model.
The team selected this protocol because it is the only one that triages individuals at baseline to biennial, rather than annual, screening if they have no lung nodules or face a very low risk. The study compared the ILST protocol with two others when prospectively evaluating the management of baseline LDCT results: the volume-based NELSON trial protocol and the diameter-based Lung-RADS v1.1.
Study Design and Results

The BC Lung Screen Trial recruited more than 2,100 individuals ages 55 to 80 (median 64) with any history of smoking who were eligible for screening according to the PLCO M2012 prediction model of 6-year lung cancer risk, applied at a risk threshold of 1.5% or greater, or according to the USPSTF 2013 criteria. Of them, 1,783 completed their first follow-up screen, presenter Renelle Myers, MD, said.
Among those participants, 54% were former smokers, 87% were white, and 52% were female. A total of 3.7% were found to have lung cancer, which, in 77% of cases, was stage I or II NSCLC.
Managed using ILST follow-up recommendations, participants were triaged into one of three groups. Those in Group 1 had a nodule risk score of less than 5% and were advised to return for routine surveillance LDCT in 12 months (Group 1A) or 24 months (Group 1B); participants in Group 2 had a risk score of 5% to 30% and were asked to return at 3 months; and individuals in Group 3 had a risk score of over 30% or were deemed high-risk by a radiologist and were referred for diagnostic work-up. The implications of using NELSON and Lung-RADS to form recommendations about screening after baseline for the same patients were determined using the largest nodule visible in each scan. Participants were followed for 30 months after their first LDCT screen.
The proportion of participants triaged to routine surveillance (Group 1) was similar between the three protocols. However, 65.7% of all the participants managed using the ILST protocol were prescribed biennial, rather than annual, follow-up, Dr. Myers said. The lung cancer rate (0.9%) and proportion of early-stage cancers (81%) with ILST were similar to those demonstrated with the NELSON (1% and 80%, respectively) and Lung-RADS protocols (1.3% and 86%, respectively).
The ILST protocol outperformed NELSON and Lung-RADS with a positive predictive value of 17% versus 11% and 13%, respectively, she said.
“The ILST lung nodule management protocol allowed 66% of the participants to have biennial follow-up instead of annual follow-up without an increase in the number and proportion of advanced lung cancers,” Dr. Myers said. “It had the lowest early recall and diagnostic referral rates compared to NELSON and Lung-RADS, and this has significant potential implications in healthcare resource utilization and cost-effectiveness.”
Weighing the Findings
Dr. Pasquinelli said that the protocol evaluated in the study “aims to utilize healthcare resources most efficiently while keeping the effectiveness of detecting lung cancer early.”
As the study’s population was primarily white, she said that the effectiveness of the ILST protocol should next be tested in Native American and Black individuals, populations with some of the highest rates of lung cancer and unique smoking patterns.
Future studies should also analyze the ILST protocol in participants selected according to the USPSTF’s 2021 criteria and the PLCO M2012 prediction model, applied at a risk threshold of 1%, she said.
Decreased False Positives Versus Delayed Diagnosis: Finding the Balance
A drawback of LDCT screening for lung cancer is the potential for false positives. The frequency of this outcome could be reduced by changing the size of the nodules that constitute a positive screen. However, increasing the nodule size threshold would result in a certain percentage of very early cancers going undiagnosed until they grew.
In a retrospective study, a team of researchers from the radiology department at the Icahn School of Medicine at Mount Sinai sought a balance between those competing factors.
Using data gleaned from the I-ELCAP database about the baseline screenings of 21,136 individuals between 2006 and 2010, the researchers calculated the rate of positive screening results that would have been recorded if increasing size thresholds had been applied. Then, they determined how many cancer diagnoses would have been delayed based on higher size thresholds combined with histology. Finally, based on the predicted size of the nodules 1 year after the baseline screen, they estimated the change in curability due to the delayed diagnoses, presenter Rowena Yip, MPH, said.
Study Findings
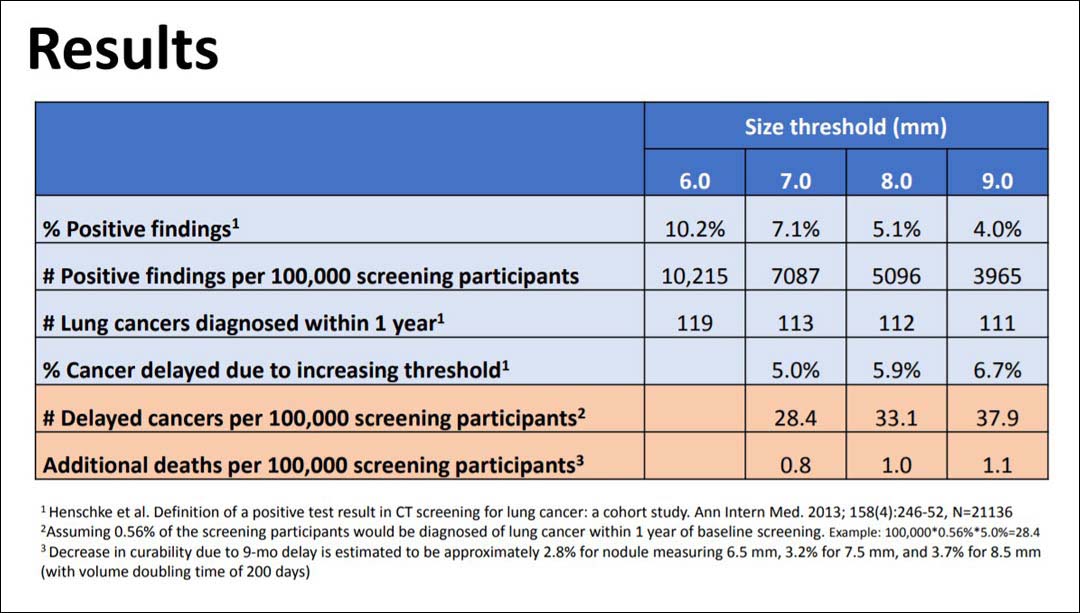
Moving from a 6 mm size threshold for the initiation of work-up to 7 mm, 8 mm, and 9 mm, the investigators found that the rate of positive screens decreased from 102.1/1000 (10.2%) to 70.9/1000 (7.1%), 51.0/1000 (5.1%), and 39.6/1000 (4.0%), respectively. They assumed that 0.56% of participants would be diagnosed with lung cancer within 1 year of baseline screening and that the higher thresholds would delay the diagnosis of 5% to 6% of cancers until the next annual screening. Based on those assumptions, they deduced that, compared with the 6 mm threshold, diagnosis would have been delayed in 28, 33, and 38 cases per 1,000 screening participants using the 7 mm, 8 mm, and 9 mm thresholds. Out of a population of 100,000 people screened, 119 cancers would have been diagnosed within 1 year using the 6 mm threshold versus 111 using the 9 mm threshold. Further, in a population of 100,000, lung cancer deaths would have increased by 0.8, 1.0, and 1.1 as the threshold increased to 7 mm, 8 mm, and 9 mm, Ms. Yip reported.
She concluded that increasing the baseline nodule size threshold from 6 mm to 9 mm would result in about 1 lung cancer death per 100,000 people screened, adding that this could make screening accessible in countries with limited resources.
“Not performing any screening at all would result in between 5,000 to 10,000 deaths per 100,000 people,” she said, “so using a high threshold still provides a huge benefit.”
Dr. Pasquinelli said that nodule size threshold is important not only because it determines what constitutes a positive LDCT screen, but because it sets the algorithm for follow-up scans and diagnostic work-up.
“Having a higher nodule size threshold may be reasonable when resources are limited,” she said. “The main point is that lung cancer screening saves lives, and it is important that countries start implementing and optimizing lung screening programs.”
Reference:
- 1. AAFP. USPSTF Recommends Lung Cancer Screening With Low-dose CT. Published July 15, 2020. Accessed Nov. 13, 2021. https://www.aafp.org/news/health-of-the-public/20200715uspstfdraftlung.html.