Dysregulation of human epidermal growth factor receptor 2 (HER2)—via HER2 amplification, mutation, or overexpression of HER2 protein—is associated with the pathogenesis of various cancers. In non-small cell lung cancer (NSCLC), HER2 dysregulation via mutation is a rare and clinically challenging entity.
Activating HER2 mutations occur in 1% to 7% of NSCLC patients, depending on their ancestry.1 The 2022 approval of the antibody-drug conjugate (ADC) trastuzumab deruxtecan marked the first targeted therapy approved for patients with HER2-mutated NSCLC.

During the second of two Presidential Symposia at the 2024 World Conference on Lung Cancer, Gerrina Ruiter, MD, PhD, Assistant Professor and pulmonologist specializing in thoracic oncology at the Netherlands Cancer Institute in Amsterdam, reviewed findings for a novel HER2 TKI—zongertinib.
“HER2 mutations are rare in NSCLC, but they are associated with poor prognosis and higher incidence of brain metastases,” Dr. Ruiter said. “Zongertinib is a new, orally administered TKI that selectively and covalently binds to the tyrosine kinase domain (TKD) of HER2 alone, while sparing wild-type EGFR, thereby avoiding unnecessary toxicities.”
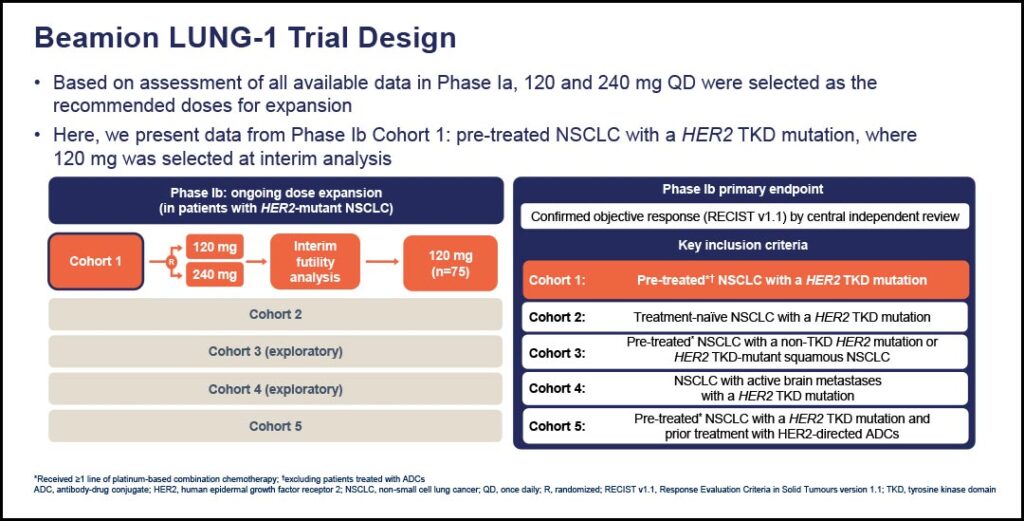
Dr. Ruiter reviewed updated findings from cohort 1 of the phase Ib Beamion LUNG-1 study, which evaluated zongertinib in pretreated patients with HER2-mutated NSCLC.
The primary endpoint, the overall response rate, confirmed by the Independent Committee Review, was met in patients at 67%, with tumor shrinkage reported per investigator assessment in 94% of patients.
Data for the duration of response and progression-free survival are not yet mature, as two-thirds of the patients were still under treatment as of the data cutoff, Dr. Ruiter said.
“We also see some encouraging preliminary intracranial activity with zongertinib. Although the numbers are smaller, a confirmed response within the brain was seen in about 33% of the patients. Most importantly, the DCR is also very high in the brain (74%),” Dr. Ruiter said.
Of the 132 patients who received 120 mg of zongertinib treatment, more than one-third (37%) had brain metastases, more than half (56%) had received one prior therapy, 16% had received two, and 28% had received three or more prior therapies.
Diarrhea and rash were the most common treatment-related adverse events, occurring in 48% (43% grade 1, 11% grade 2) and 24% (19% grade 1, 8% grade 2), respectively. Adverse events (AEs) leading to dose reduction occurred in 11% of patients; 3% of patients discontinued treatment due to AEs.
“Zongertinib was very well tolerated, with no deaths attributable to treatment and a low incidence of dose reductions and treatment discontinuations,” Dr. Ruiter said.
She added that the US FDA has granted zongertinib Breakthrough Therapy Designation and that the phase III Beamion LUNG-2 randomized study comparing zongertinib to standard of care in HER2-mutated NSCLC is currently enrolling.
Looking more broadly at the clinical challenge of managing HER2-mutated NSCLC, discussant Chee Lee, MBBS, PhD, Professor of Oncology at the University of Sydney, and Associate Editor of JTO Clinical and Research Reports noted, “I often think of HER2-mutated lung cancer as the ‘poor cousin’ of EGFR-mutated lung cancer, as there have been no effective targeted therapies for this molecular subset,” he said. “Clinical development of BAY 2927088 (see Study Results Support Continued Research of HER2-Targeted TKI) and zongertinib represent an important achievement in HER2 mutation-positive NSCLC. These treatments provide important hope for our patients; however, the roadmap towards routine clinical use requires more consideration of optimized dosing to improve tolerability.”
Prof. Lee also noted that ongoing translational research can shed light on mechanisms of acquired resistance to kinase inhibitors. He said that such investigations can also help design trials, based on rational combinations and appropriate sequencing of targeted therapies.
References
- 1. Ren S, Wang J, Ying J, et al. Consensus for HER2 alterations testing in non-small-cell lung cancer [published correction appears in ESMO Open. 2022 Jun;7(3):100482. doi: 10.1016/j.esmoop.2022.100482]. ESMO Open. 2022;7(1):100395. doi:10.1016/j.esmoop.2022.100395










