A novel combination of benmelstobar, anlotinib, and etoposide/carboplatin chemotherapy (EC) has resulted in the longest overall survival seen to date in extensive-stage small cell lung cancer (ES-SCLC). The combination yielded a median survival of 19.3 months compared to 11.9 months for placebo + EC (HR 0.61, p=0.0002) in a multicenter phase III trial presented on Sunday, September 10, at the 2023 World Conference on Lung Cancer in Singapore. The study was presented during the oral abstract session, “Advancing Immunotherapy in ES-SCLC,” which can be watched on-demand by registered attendees through December 31, 2023.
“SCLC is a recalcitrant disease, and these results are extremely encouraging,” said Ying Cheng, MD, First-Class Professor and Chief Physician in Thoracic Oncology, Jilin Cancer Hospital, Changchun, China. “The combination of benmelstobar, anlotinib, and chemotherapy achieved historically long overall survival and progression-free survival in ES-SCLC. This approach demonstrates a significant survival extension over chemotherapy alone and has a tolerable safety profile.”

Oral Abstract 01: Advancing Immunotherapy in ES-SCLC
Benmelstobart with Anlotinib plus Chemotherapy as First-line Therapy for ES-SCLC: A Randomized, Double-blind, Phase III Trial
Five-Year Survival in Patients with ES-SCLC Treated with Atezolizumab in IMpower133: Imbrella a Extension Study Results
Phase I Dose Escalation Trial Of The DLL3/CD3 Igg-Like T Cell Engager BI 764532 In Patients with DLL3+ Tumors: Focus on SCLC
First-Line Chemotherapy With or Without Tislelizumab for Extensive-Stage Small Cell Lung Cancer: RATIONALE-312 Phase 3 Study
ES-SCLC is a challenging malignancy to treat with modest long-term survival benefits with the addition of immunotherapy. The SCLC tumor microenvironment is characterized by immunosuppression, neoangiogenesis, and vascularization, all of which can inhibit immune cell infiltration and could be targets for improving outcomes.
Dr. Cheng said that the combination of benmelstobar, a newly developed PD-L1 inhibitor, anlotinib, a tyrosine kinase inhibitor targeting vascular endothelial growth factor receptors 2 and 3, and standard EC is an attempt to reprogram the tumor microenvironment and normalize tumor vasculature to promote immune cell infiltration.
Researchers enrolled a total of 738 patients across 72 centers in China. The median age of patients was about 62, 85% were male, and more than 20% reported never smoking. Nearly all patients had ES-SCLC, about 90% stage IV and 10% stage III. Brain, liver, and bone metastases occurred in 10%, 32%, and 28% of patients respectively.
Results here in ES-SCLC appear to be unprecedented, but the inclusion of more than 20% of patients with no history of tobacco exposure makes this population highly atypical compared to those we have historically seen in ES-SCLC trials.
—Corey J. Langer, Editor
Of the cohort, 246 patients were randomly assigned to benmelstobar + anlotinib + chemotherapy (EC), 245 to placebo + anlotinib + EC, and 247 to combination placebo + EC. There were no significant differences in baseline characteristics between the three groups.
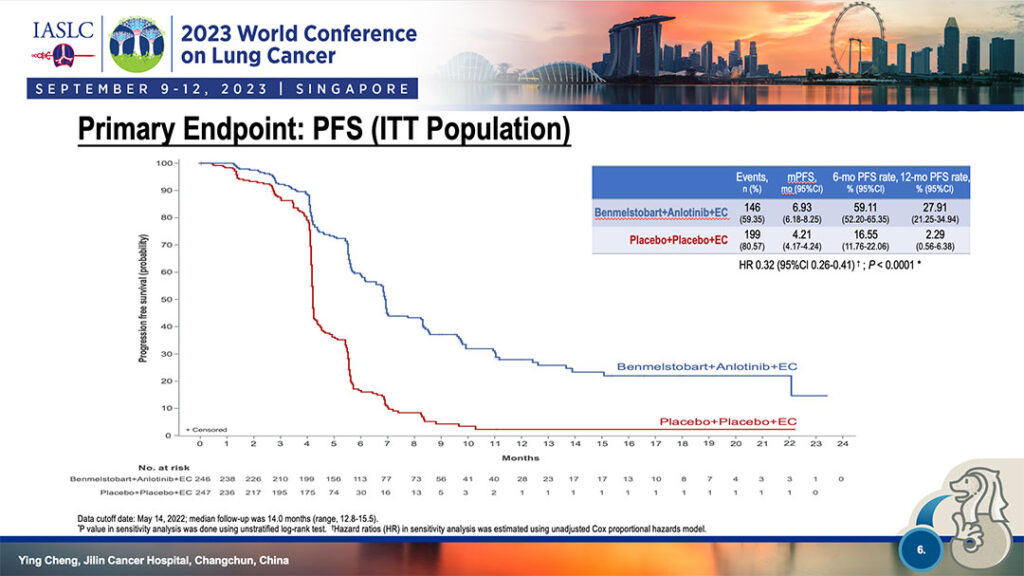
Median progression free survival was 6.93 months for the four-drug group vs. 4.21 months for the EC-only group (HR 0.32, p<0.0001). The 6-month PFS rate was 59.11% for the four-drug combination and 16.55% for EC-only. At 12 months, PFS was 27.91% for the four-drug combination and 2.29% for EC-only. (See Fig. 1)
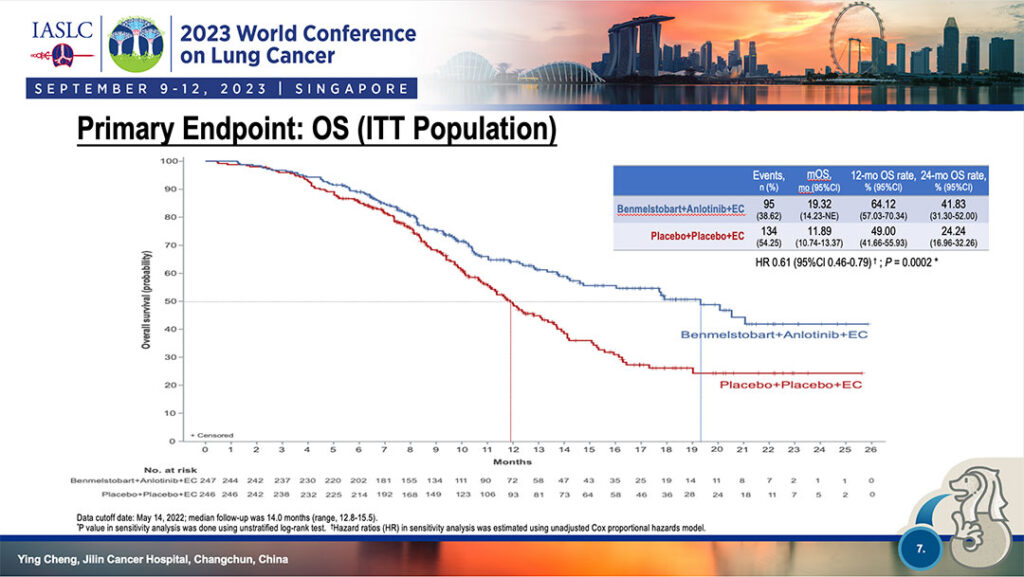
Median overall survival was similarly separated, 64.12% at 12 months for four-drug combination versus 49.0% for EC-only and 41.83% at 24 months for the four-drug therapy versus 24.24% for EC-only. (See Fig. 2)
OS benefits of benmelstobar + anlotinib + chemotherapy were somewhat reduced in patients with brain metastases and in patients with a history of tobacco exposure. PFS benefits were reduced in patients with brain metastases and limited-stage disease.
The safety profile of triple therapy was manageable and tolerable, Dr. Yeng reported, with grade ≥3 treatment-related adverse events in 93.1% of patients compared to 87.0% of EC-only patients. The most common grade ≥3 TRAEs were decreased neutrophils, platelet, and white blood cell counts. A subset of patients reported immune-related adverse events.
“The addition of an anti-angiogenic agent to immunochemotherapy in the first-line treatment of ES-SCLC resulted in the historically longest PFS and OS,” Dr. Yeng said. “These results support the use of immunochemotherapy plus anti-angiogenesis as a new treatment option for this very difficult to treat population of patients.”