The original PACIFIC trial irrevocably altered the treatment paradigm for patients with stage III non-small-cell lung cancer (NSCLC) and no disease progression after concurrent chemoradiotherapy. The PACIFIC regimen—concurrent chemoradiotherapy followed by 12 months of consolidation durvalumab—resulted in a 5-year overall survival and progression-free survival improvement compared to standard chemoradiation alone.1

However, as investigator Jeffrey D. Bradley, MD, said on March 21 during the 2024 European Lung Cancer Congress (ELCC), up to a third of patients may experience disease progression or adverse events during the chemoradiation phase and be ineligible to receive durvalumab as consolidation therapy.
Dr. Bradley, Professor in the Department of Radiation Oncology at University of Pennsylvania, Philadelphia, said preclinical evidence suggested using immunotherapy (IO) concurrently may help circumvent disease progression and offer additional benefit.
“It may have a synergistic effect with radiation therapy which could provide an opportunity for some patients to benefit during chemoradiation, and more, to be eligible to receive IO or increase the rate or depth of response leading to improved clinical benefit,” he said.
With that in mind, PACIFIC-2 became the first phase III study designed to assess the efficacy of concurrent IO—in this case durvalumab—plus chemoradiation followed by consolidation durvalumab in unresectable stage III NSCLC. Dr. Bradley presented late-breaking results from PACIFIC2 during ELCC 2024 in Prague, Czech Republic.
PACIFIC-2 was a randomized, double-blind, placebo-controlled multi-center global study. Participants with locally advanced stage III NSCLC and good performance statuses of 0 to 1 were randomized 2 to 1 to receive the experimental arm—durvalumab with chemoradiation followed by consolidation durvalumab until disease progression—or placebo and chemoradiation followed by placebo until progression. Dr. Bradley pointed out that the patients enrolled in PACIFIC-2 were primarily from Asia, Eastern Europe, and Central and South America, while the original PACIFIC trial had more patients from Europe and North and South America and fewer from Asia. The primary endpoint was progression-free survival (PFS). Key secondary endpoints included overall survival and safety.
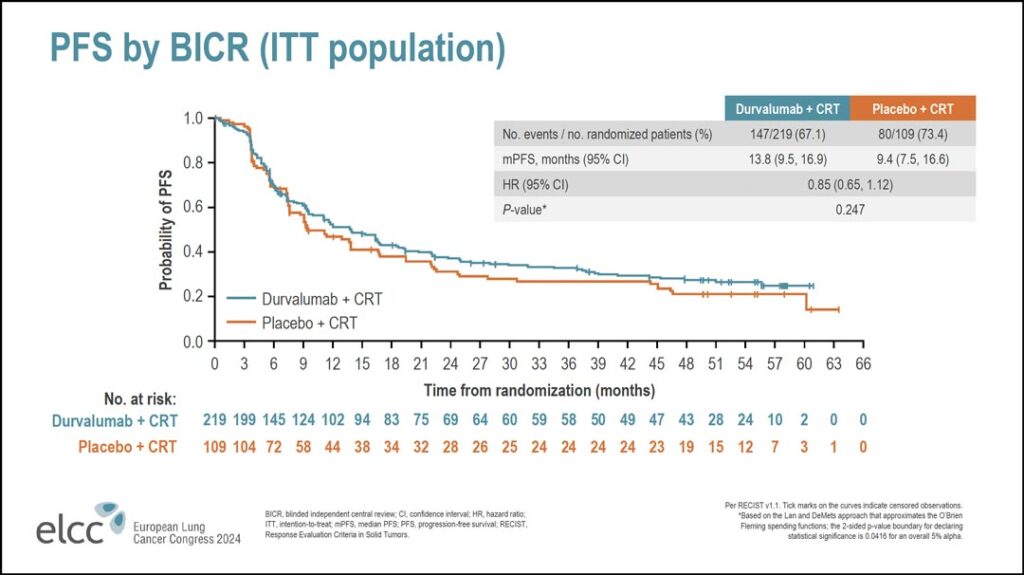
Looking at the progression-free survival data (see Figure 1), Dr. Bradley said the first thing to note is that in the first 6 months of treatment, during the chemoradiation phase, there was no difference between the two arms.
“There’s no early uplift with the addition of immunotherapy,” he said. “There is a numerical improvement in the hazard ratio for PFS, but that did not reach statistical significance. The median progression free survival in the durvalumab arm was 13.8 months compared to 9.4 months in the placebo arm. That was associated with hazard ratio of 0.85 with a nonsignificant P value.”
Looking at PFS more closely, Dr. Bradley said most of the subgroups analyzed have a hazard ratio of less than one, but none reached statistical significance. The populations that appeared to perform better with concurrent durvalumab included women, patients younger than 65 years of age, patients from Europe, and patients with smaller tumor volumes.
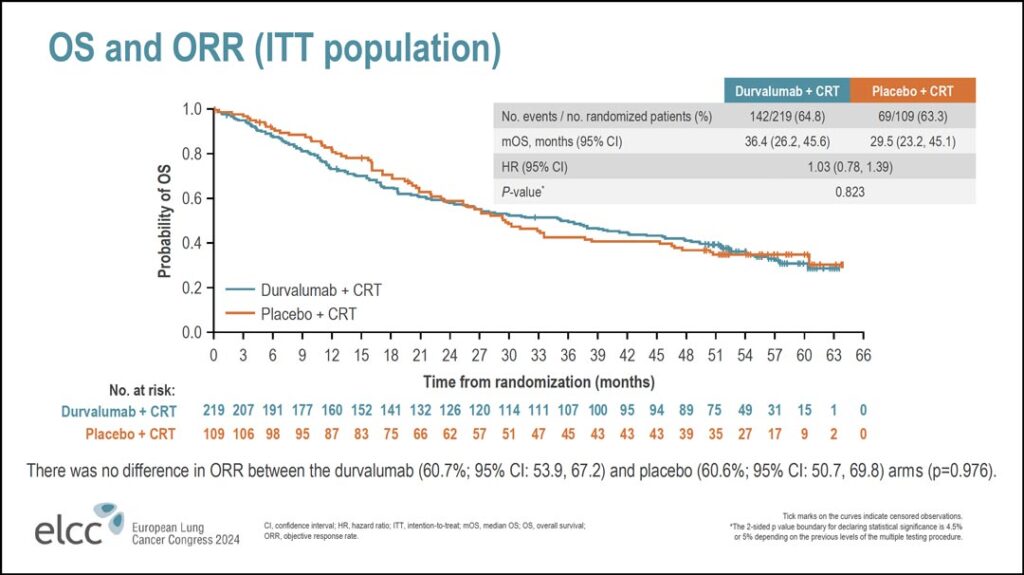
The overall survival data were similar (see Figure 2).
“Again, there’s no difference in overall survival,” Dr. Bradley said. “The hazard ratio here is 1.03. The median overall survival in the durvalumab arm was 36.4 months versus 29.5 months with placebo. No significant difference. If you look at overall response rate, it was the same in both arms; 60.7% in the durvalumab arm versus 60.6% in the placebo arm.”
There were no key differences seen in overall survival in the subgroup analyses, he said. As with PFS, women, younger patients, and patients from Europe appeared to derive greater benefit from concurrent durvalumab than others.
During his presentation, Dr. Bradley did not theorize why the results did not show a synergistic benefit with concurrent IO and chemoradiotherapy that many had hoped for. However, in reviewing the baseline patient characteristics, he pointed out some subgroups may be subjects for further investigation.
The median age of patients was 63 years in both arms, and most patients were male—approximately 75% in both arms. However, there were other demographics of note upon closer inspection.
“This study took place before we tested for EGFR routinely, so we do have a higher number of patients with unknown EGFR status in this patient population; 45.7% in the durvalumab arm and 40% in the placebo arm,” he said. “Note that we also had more T4 tumors than you would expect: 57.5% in the durvalumab arm and 48.6% in the placebo arm. Likewise, we had more N2 patients.
“Additionally, the durvalumab arm included more patients with a performance status of 1, more patients with squamous histology, more patients with PDL1 expression less than 1%, and, as I said, more patients with T4 tumors.”
Dr. Bradley also shared data on the key secondary endpoint of safety.
Most patients—approximately 90% in both arms—completed chemoradiation. If they discontinued radiation, the most common cause was an adverse event: 9.2% in the experimental arm versus 4.6% in the placebo arm. While most patients finished the treatment, the rate of adverse events leading to discontinuation was 26.6% in the durvalumab arm verses 13.8% in the placebo arm.
“Looking at adverse events, they’re very similar between arms; the maximum grade 3 and 4 events were 53.4% in the durvalumab arm versus 59% in the placebo arm,” Dr. Bradley said. “Most of these were anemia or lymphopenia and there was no difference between the two arms. We did have more deaths in the durvalumab arm, 13.7% versus 10.2%. If you break it down by time—and we broke it down by 0 to 4 months, 4 to 16 months, and greater than 16 months—0 to 4 months approximates roughly the duration until our first scan after treatment. We saw more events in that time frame than the other two time periods. We also saw more treatment discontinuations in that early time frame: 14.2% versus 5.6%. But this did not appear to be due to pneumonitis. If you look at grade 3 pneumonitis or radiation pneumonitis, it was similar between the two arms: 4.6% in the durvalumab arm and 5.6% in the placebo arm.
“Looking at this another way, grade 3 to 4 adverse events and adverse events leading to death were overall the same between groups except during that 0- to 4-month timeframe. There we had more grade 3 or 4 events in the durvalumab group, 57% versus 53%, and we had more deaths in the durvalumab group, 6.8% versus 4.6%.”
He concluded that immunotherapy and concurrent chemoradiation followed by a consolidation immunotherapy did not significantly improve overall or progression free survival in patients with stage III, unresectabe non-small cell lung cancer. Thus, concurrent chemoradiation followed by consolidation durvalumab, i.e. the PACIFIC regimen, remains standard of care for patients with unresectable stage III non-small cell lung cancer, he said.
References
- 1. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer [published correction appears in J Clin Oncol. 2022 Jun 10;40(17):1965]. J Clin Oncol. 2022;40(12):1301-1311. doi:10.1200/JCO.21.01308