
For more than a decade, thoracic oncologists have made significant progress in the treatment of patients with non-small cell lung cancer (NSCLC). However, as discussed during an educational session at the 2022 North America Conference on Lung Cancer—which took place September 23-25 in Chicago—the 5-year overall survival (OS) rates for patients with localized stage II or locally advanced stage III NSCLC remain low.
“Despite curative surgery, approximately 30% to 55% of patients develop disease recurrence and ultimately die of their disease,” said Tina Cascone, MD, PhD, as she presented a rationale for the use of immunotherapy (IO) in resectable NSCLC during NACLC.
Dr. Cascone, who is an assistant professor in the Department of Thoracic/Head and Neck Medical Oncology at the University of Texas MD Anderson Cancer Center in Houston, outlined several other considerations in support of IO:
- Neoadjuvant or adjuvant chemotherapy is recommended for patients with high risk of recurrence but provides only a modest (~5%) improvement in 5-year OS.
- In localized disease, the immune system may be more intact and clonal resistance is minimal, suggesting an optimal patient response to IO.
- In murine models of cancer, tumor PD-L1 facilitates metastases and neoadjuvant combined immune checkpoint inhibitors (ICIs) are superior to adjuvant therapy at improving survival and enhancing antitumor immunity
While data supports the use of immunotherapy in NSCLC, questions remain about when to use ICI, Dr. Cascone said during her lecture “Neoadjuvant or Adjuvant Therapy in NSCLC: What’s the Impact?”
Neoadjuvant Therapy
During her talk, Dr. Cascone reviewed the science—including data from CheckMate 816 and NEOSTAR—that support the use of neoadjuvant IO.
“With neoadjuvant therapy, we may be able to activate the immune system when the primary tumor is intact, clonal resistance is minimal, and neoantigens are present,” she said. “We can also treat micrometastases at the earliest point and assess treatment efficacy prior to surgery.
“Perhaps most importantly, we can evaluate biomarkers and surrogate endpoints of clinical efficacy, including pCR (pathologic complete response). CheckMate 816 really shed light on the importance of pCR as a surrogate of neoadjuvant clinical benefit and benefit even for those without pCR, but we’re waiting for guidance from the FDA (US Food and Drug Administration) on this surrogate.”
Adjuvant Therapy
While many in thoracic oncology have been interested in recent positive data from neoadjuvant trials, evidence remains to support adjuvant IO. Dr. Cascone outlined three key benefits to adjuvant therapy, including reduced time to surgery, and it enables longer treatment duration.
“More importantly, we may be able to restore antitumor immunity that may have been impaired by surgery, which has been shown to have an immunosuppressive effect,” she said. “We have a new standard of care with Impower010. In IMpower010, adjuvant atezolizumab demonstrated a disease-free survival (DFS) benefit among patients with resected stage II to stage IIIA NSCLC. As we saw in the update presented at World Lung (Conference on Lung Cancer), the data showed improved survival with even greater benefit in patients with high PD-1 status.”
Conversely, the PEARLS/KEYNOTE-091 study, which tested adjuvant pembrolizumab in patients with stage IB to stage IIIA NSCLC, also demonstrated an improvement in DFS, but it was not as significant in patients with PD-L1 >50%, Dr. Cascone said.
“Perhaps suggesting we need to do more work to understand the clinical, pathologic, and molecular characteristics that are impacting these results,” she said.
Perioperative Therapy
Finally, Dr. Cascone looked at the evidence for perioperative therapy.
“With perioperative therapy, we can offer continuous treatment throughout the surgical setting,” she said. “We can enhance micrometastatic disease killing and antitumor immunity, and perhaps we can have greater impact on event-free survival and overall survival without concerning toxicity. Ongoing and recently completed perioperative trials will answer that.” (see Fig. 1)
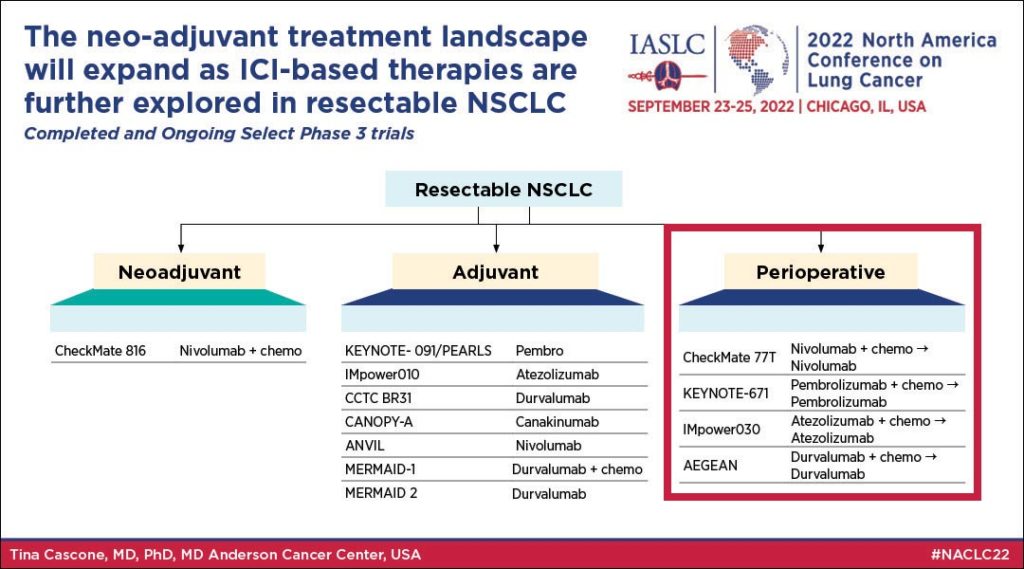
Dr. Cascone said ongoing trials will determine whether thoracic oncologists need an adjuvant immunotherapy phase following a neoadjuvant phase of immunotherapy plus chemotherapy administration.
“Is neoadjuvant better than adjuvant?” she said in conclusion. “We really don’t have an answer from phase 3 randomized trials. We shouldn’t be doing cross trial comparisons. We can extrapolate from melanoma data recently presented at ESMO. We have an answer from mouse models. But in the clinical setting, we really don’t have an answer to this question. We do know that combined, multiple immune pathway inhibition leads to higher major pathologic response and cPR compared to chemotherapy and ICI monotherapy.





