
Andrew McKenzie, PhD
Immune checkpoint inhibitors (ICIs) have revolutionized cancer care. However, current biomarker strategies are only able to predict response to ICIs in a minority of patients. The advent of agents targeting different aspects of the cancer immune system requires more sophisticated patient selection techniques to maximize tumor response to these novel immunotherapies.1,2
Current patient selection strategies for approved ICIs include immunohistochemical staining of PD-L1, polymerase chain reaction (PCR) or next-generation sequencing (NGS) for microsatellite instability (MSI); immunohistochemical staining or PCR for DNA mismatch repair deficiency (dMMR); and NGS for tumor mutation burden (TMB).
However, these techniques predict response to ICIs for only a minority of patients, and methods for detecting these biomarkers can be complex and tissue- or ICI-specific.3
Recently, NGS has been used to detect the somatic mutation landscape and calculate tumor mutation burden (TMB). The FDA recently approved tumor mutation burden as a predictive biomarker for response to immunotherapies.4,5 This strategy, however, is imperfect. The overall response rate to pembrolizumab in patients harboring high TMB was 29% in the KEYNOTE-158 study.4
Further confounding patient selection, TMB values can vary between vendors and between the type of sample being analyzed—i.e. tissue samples versus circulating tumor DNA (ctDNA).6,7 Similarly, dMMR is a robust marker for predicting response to ICIs, but only a minority of cancers have dMMR and many MMR-proficient tumors still respond to ICI treatment.8,9
The complexity outlined above coupled with the relatively low predictive value of these biomarkers demonstrates the need for more robust tools to identify patients who are likely to respond to ICIs and, just as importantly, spare patients the time, expense, and toxicity of trying therapies from which they will not derive benefit.
We have initiated work at Sarah Cannon Research Institute, Nashville, Tennessee, to elucidate molecular signatures from NGS that correlate with response to ICIs. To do this, we rely on NGS performed as a part of standard of care from our network of community-based general medical oncologists. Comprehensive genomic profiling via NGS is now recommended for many tumor types for identifying patients who might respond to targeted therapies. We aim to expand the utility of these tests to identify patients who may be more likely to respond to ICIs.10
There is precedent for this line of investigation as mutations in DNA damage repair pathway genes (including POLD1, POLE, and MSH2) in non-small-cell lung cancers (NSCLC) harboring TMB have shown response to ICIs. Conversely, NSCLC tumors harboring mutations in STK11 demonstrate a lack of response to single-agent ICI treatment.11,12,13
Predictably, in our analysis, different tumors respond differently to ICI treatment. For example, we’ve seen longer overall survival with melanomas and lung cancers compared to non-colon GI, colorectal, and breast cancers.
Somewhat surprisingly, it appears overall survival on ICIs could be increased by personalizing treatment based on mutations in certain genes. For instance, colorectal tumors harboring mutations in NOTCH1 had a nearly 5-fold increase in median overall survival compared to their NOTCH1 wild-type counterparts.
Similarly in NSCLC, tumors harboring mutations in LRP1B had a 1.4-fold increase in median overall survival compared to their LRP1B wild-type counterparts (Fig. 1).
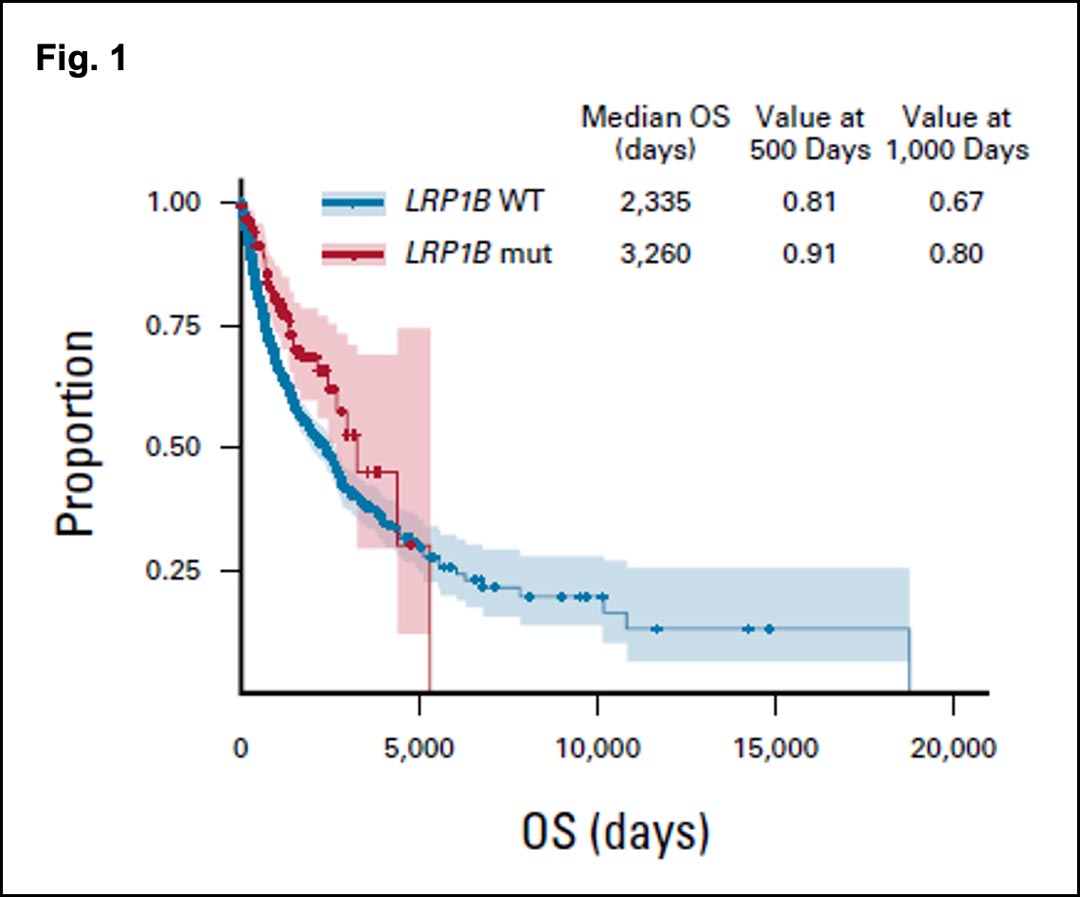
Adjusted overall survival in patients receiving immune checkpoint inhibitors with non-small-cell lung cancer. Light red and light blue lines represent mutant and wild type, respectively. Schlauch D, Fu X, Jones SF, et al. Tumor-Specific and Tumor-Agnostic Molecular Signatures Associated With Response to Immune Checkpoint Inhibitors. JCO Precis Oncol.
In our research, we have observed that NSCLCs harbor numerous genes numerous genes with mutations that are associated with a better response to ICIs. Interestingly, mutations in genes associated with growth factor or mitogen signaling (EPHA3, GNAS, ERBB2, NF1, and ALK) generally had prolonged response to ICIs with EGFR being the exception. EGFR mutations were associated with lack of response to ICIs, as has been previously shown.
For each tumor type analyzed, we were able to elucidate mutations in certain genes that corresponded with longer overall survival on ICIs than their wild-type counterparts—indicating that there may be genomic signatures

Andrew McKenzie, PhD
Immune checkpoint inhibitors (ICIs) have revolutionized cancer care. However, current biomarker strategies are only able to predict response to ICIs in a minority of patients. The advent of agents targeting different aspects of the cancer immune system requires more sophisticated patient selection techniques to maximize tumor response to these novel immunotherapies.1,2
Current patient selection strategies for approved ICIs include immunohistochemical staining of PD-L1, polymerase chain reaction (PCR) or next-generation sequencing (NGS) for microsatellite instability (MSI); immunohistochemical staining or PCR for DNA mismatch repair deficiency (dMMR); and NGS for tumor mutation burden (TMB).
However, these techniques predict response to ICIs for only a minority of patients, and methods for detecting these biomarkers can be complex and tissue- or ICI-specific.3
Recently, NGS has been used to detect the somatic mutation landscape and calculate tumor mutation burden (TMB). The FDA recently approved tumor mutation burden as a predictive biomarker for response to immunotherapies.4,5 This strategy, however, is imperfect. The overall response rate to pembrolizumab in patients harboring high TMB was 29% in the KEYNOTE-158 study.4
Further confounding patient selection, TMB values can vary between vendors and between the type of sample being analyzed—i.e. tissue samples versus circulating tumor DNA (ctDNA).6,7 Similarly, dMMR is a robust marker for predicting response to ICIs, but only a minority of cancers have dMMR and many MMR-proficient tumors still respond to ICI treatment.8,9
The complexity outlined above coupled with the relatively low predictive value of these biomarkers demonstrates the need for more robust tools to identify patients who are likely to respond to ICIs and, just as importantly, spare patients the time, expense, and toxicity of trying therapies from which they will not derive benefit.
We have initiated work at Sarah Cannon Research Institute, Nashville, Tennessee, to elucidate molecular signatures from NGS that correlate with response to ICIs. To do this, we rely on NGS performed as a part of standard of care from our network of community-based general medical oncologists. Comprehensive genomic profiling via NGS is now recommended for many tumor types for identifying patients who might respond to targeted therapies. We aim to expand the utility of these tests to identify patients who may be more likely to respond to ICIs.10
There is precedent for this line of investigation as mutations in DNA damage repair pathway genes (including POLD1, POLE, and MSH2) in non-small-cell lung cancers (NSCLC) harboring TMB have shown response to ICIs. Conversely, NSCLC tumors harboring mutations in STK11 demonstrate a lack of response to single-agent ICI treatment.11,12,13
Predictably, in our analysis, different tumors respond differently to ICI treatment. For example, we’ve seen longer overall survival with melanomas and lung cancers compared to non-colon GI, colorectal, and breast cancers.
Somewhat surprisingly, it appears overall survival on ICIs could be increased by personalizing treatment based on mutations in certain genes. For instance, colorectal tumors harboring mutations in NOTCH1 had a nearly 5-fold increase in median overall survival compared to their NOTCH1 wild-type counterparts.
Similarly in NSCLC, tumors harboring mutations in LRP1B had a 1.4-fold increase in median overall survival compared to their LRP1B wild-type counterparts (Fig. 1).

Adjusted overall survival in patients receiving immune checkpoint inhibitors with non-small-cell lung cancer. Light red and light blue lines represent mutant and wild type, respectively. Schlauch D, Fu X, Jones SF, et al. Tumor-Specific and Tumor-Agnostic Molecular Signatures Associated With Response to Immune Checkpoint Inhibitors. JCO Precis Oncol.
In our research, we have observed that NSCLCs harbor numerous genes numerous genes with mutations that are associated with a better response to ICIs. Interestingly, mutations in genes associated with growth factor or mitogen signaling (EPHA3, GNAS, ERBB2, NF1, and ALK) generally had prolonged response to ICIs with EGFR being the exception. EGFR mutations were associated with lack of response to ICIs, as has been previously shown.
For each tumor type analyzed, we were able to elucidate mutations in certain genes that corresponded with longer overall survival on ICIs than their wild-type counterparts—indicating that there may be genomic signatures beyond tumor mutation burden and microsatellite instability that predict response to ICIs. In fact, even in the high MSI and high TMB tumors that were analyzed, mutations in numerous genes, including KMT2D and POLD1, significantly prolonged the time to ICI resistance.
These results indicate that while MSI and TMB may predict response to ICI in some patients, other, more robust molecular signatures may exist that predict deeper responses to ICI in more patients. Importantly, our work was performed retrospectively on NGS tests that were ordered as a part of standard of care. Therefore, these potential signatures may already be in the patient records and available to inform treatment selection without additional testing.
Further testing in the context of prospective trials will be needed to confirm our findings.
References
- 1. Yuan J, Hegde PS, Clynes R, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4(1):3. doi:10.1186/s40425-016-0107-3
- 2. Shindo Y, Hazama S, Tsunedomi R, Suzuki N, Nagano H. Novel Biomarkers for Personalized Cancer Immunotherapy. Cancers (Basel). 2019;11(9):1223. doi:10.3390/cancers11091223
- 3. Spencer KR, Wang J, Silk AW, Ganesan S, Kaufman HL, Mehnert JM. Biomarkers for Immunotherapy: Current Developments and Challenges. Am Soc Clin Oncol Educ B. 2016;(36):e493-e503. doi:10.1200/EDBK_160766
- 4. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353-1365. doi:10.1016/S1470-2045(20)30445-9
- 5. Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598-2608. doi:10.1158/1535-7163.MCT-17-0386
- 6. Sturgill EG, Misch A, Jones CC, et al. Discordance in Tumor Mutation Burden from Blood and Tissue Affects Association with Response to Immune Checkpoint Inhibition in Real-World Settings. Oncologist. 2022;27(3):175-182. doi:10.1093/oncolo/oyab064
- 7. Merino DM, McShane LM, Fabrizio D, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother cancer. 2020;8(1). doi:10.1136/jitc-2019-000147
- 8. Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol Res. 2019;7(10):1570-1573. doi:10.1158/2326-6066.CIR-19-0149
- 9. André T, Shiu K-K, Kim TW, et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N Engl J Med. 2020;383(23):2207-2218. doi:10.1056/NEJMoa2017699
- 10. Schlauch D, Fu X, Jones SF, et al. Tumor-Specific and Tumor-Agnostic Molecular Signatures Associated With Response to Immune Checkpoint Inhibitors. JCO Precis Oncol. 2021;(5):1625-1638. doi:10.1200/PO.21.00008
- 11. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi:10.1126/science.aaa1348
- 12. Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2017;168(3):542. doi:10.1016/j.cell.2017.01.010
- 13. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8(7):822-835. doi:10.1158/2159-8290.CD-18-0099
predict response to ICIs. In fact, even in the high microsatellite instability and high tumor mutation burden tumors that were analyzed, mutations in numerous genes, including KMT2D and POLD1, significantly prolonged the time to ICI resistance.
These results indicate that while microsatellite instability and tumor mutation burden may predict response to ICI in some patients, other, more robust molecular signatures may exist that predict deeper responses to ICI in more patients. Importantly, our work was performed retrospectively on NGS tests that were ordered as a part of standard of care. Therefore, these potential signatures may already be in the patient records and available to inform treatment selection without additional testing.
Further testing in the context of prospective trials will be needed to confirm our findings.
References
- 1. Yuan J, Hegde PS, Clynes R, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4(1):3. doi:10.1186/s40425-016-0107-3
- 2. Shindo Y, Hazama S, Tsunedomi R, Suzuki N, Nagano H. Novel Biomarkers for Personalized Cancer Immunotherapy. Cancers (Basel). 2019;11(9):1223. doi:10.3390/cancers11091223
- 3. Spencer KR, Wang J, Silk AW, Ganesan S, Kaufman HL, Mehnert JM. Biomarkers for Immunotherapy: Current Developments and Challenges. Am Soc Clin Oncol Educ B. 2016;(36):e493-e503. doi:10.1200/EDBK_160766
- 4. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353-1365. doi:10.1016/S1470-2045(20)30445-9
- 5. Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598-2608. doi:10.1158/1535-7163.MCT-17-0386
- 6. Sturgill EG, Misch A, Jones CC, et al. Discordance in Tumor Mutation Burden from Blood and Tissue Affects Association with Response to Immune Checkpoint Inhibition in Real-World Settings. Oncologist. 2022;27(3):175-182. doi:10.1093/oncolo/oyab064
- 7. Merino DM, McShane LM, Fabrizio D, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother cancer. 2020;8(1). doi:10.1136/jitc-2019-000147
- 8. Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol Res. 2019;7(10):1570-1573. doi:10.1158/2326-6066.CIR-19-0149
- 9. André T, Shiu K-K, Kim TW, et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N Engl J Med. 2020;383(23):2207-2218. doi:10.1056/NEJMoa2017699
- 10. Schlauch D, Fu X, Jones SF, et al. Tumor-Specific and Tumor-Agnostic Molecular Signatures Associated With Response to Immune Checkpoint Inhibitors. JCO Precis Oncol. 2021;(5):1625-1638. doi:10.1200/PO.21.00008
- 11. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi:10.1126/science.aaa1348
- 12. Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2017;168(3):542. doi:10.1016/j.cell.2017.01.010
- 13. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8(7):822-835. doi:10.1158/2159-8290.CD-18-0099





