As an increasing number of immunotherapy treatments find their way into the front-line treatment of solid tumors, including lung cancer, clinicians are often faced with a lack of options when choosing systemic therapies after chemoimmunotherapy.
During the Education Session, “Choosing Systemic Therapies After Chemoimmunotherapy in NSCLC” three speakers provided updates on the current second-line options for patients with non-squamous, squamous, and SCLC.
Non-Squamous
Virote Sriuranpong, MD, PhD, of Chulalongkorn University & The King Chulalongkorn Memorial Hospital, Thailand, opened the session with a discussion of systemic therapy options after chemoimmunotherapy in non-squamous NSCLC.
Patients with non-squamous disease currently have several first-line treatment options including anti-PD-1/PD-L1 monotherapy, combination anti-PD-1/L1 plus anti-CTLA4, chemotherapy plus anti-PD-1/L1, or chemotherapy plus anti-PD-1/PD-L1 plus antiangiogenesis combination.
“This makes things complicated, especially when considering the mechanism of resistance,” Dr. Sriuranpong said. “One has to ask if the patient is resistant to the immune checkpoint inhibitor, the chemotherapy, the anti-angiogenic drug or all of them?”
Current National Comprehensive Cancer Network (NCCN) guidelines show that after failure of first-line systemic therapy patients have four treatment options regardless of prior immunotherapy exposure: docetaxel, pemetrexed, gemcitabine, or ramucirumab plus docetaxel.
“These are limited options available to the patient resistance to chemoimmunotherapy,” Dr. Sriuranpong said.
However, there are some reports of patients responding to subsequent chemotherapy treatment after immunotherapy in advanced NSCLC, Dr. Sriuranpong said. For example, a study from Korea showed that when chemotherapy was used as salvage after immunotherapy response rates seemed to be better compared with the last chemotherapy administered before immunotherapy.1 However, the progression-free survival (PFS) after both approaches appeared to be similar.
There are a variety of mechanisms of resistance to immune checkpoint inhibitors that have been identified including insufficient antigen presentation and recognition, insufficient T cell activation, absence of T cells from the tumor microenvironment, upregulation of immunosuppressive markers, decreased sensitivity to IFN-y signaling, and immune checkpoint markers.
When researchers try to combine immunotherapy drugs like anti-CTLA4 and anti-PD-1 inhibitors, they must think about both safety and toxicity.
“We know that adding anti-CTLA4 has more toxicity but also may enhance efficacy,” Dr. Sriuranpong said. “But other agents seem to have a bit more safety and add more space to add on like Lag-3, Tim-3, or TIGIT.”
An example of the idea that more does not always lead to better outcomes is the KEYNOTE 598 study of previously untreated stage IV NSCLC that randomly assigned patients to pembrolizumab with or without ipilimumab.2 The study did not meet its primary endpoint.
Another first-line study exploring combination treatments is the CITYSCAPE trial that randomly assigns patients to tiragolumab plus atezolizumab or atezolizumab alone.3 In this setting, the patients had to have PD-L1 tumor proportion score (TPS) of greater than 1%. The combination treatment led to enhanced response and longer PFS.
In the relapsed setting, another study looked at the anti-TIGIT vibostolimab in combination with pembrolizumab.4 Most patients had prior treatment with nivolumab or pembroliumab. The combination was more active compared with vibostolimab monotherapy. However, Dr. Sriuranpong said it was not at the level that he could say it was encouraging.
The VEGF pathway is another pathway that may be useful in the relapsed setting. The Vargado study looked at safety and efficacy of nintedanib and docetaxel in patients with lung adenocarcinoma after treatment with immune checkpoint inhibitors.5 Encouraging results showed a 58% partial response rate with disease control rate of 83%.
“There are still limited data available for second-line therapy after resistance to immune checkpoint inhibitors in nonsquamous NSCLC,” Dr. Sriuranpong said. “This is really an unmet need.”
Squamous Cell
The second speaker, Claudio Martin, of Instituto Alexander Fleming, and Hospital de Rehabilitacion Respiratoria Maria Ferrer Buenos, Aires, Argentina, gave an update on systemic therapies available after chemoimmunotherapy failure in squamous cell carcinoma (SCC).
Nowadays the standard treatment for SCC includes chemotherapy followed by immunotherapy, immunotherapy alone for PD-L1 high expression followed by chemotherapy, or chemoimmunotherapy.
“After that there are no randomized trials in these scenarios,” Dr. Martin said. “What do we do next? Unfortunately, we have to ‘visit the museum.’”
Looking at ESMO or NCCN guidelines besides the immunotherapy drugs approved for use in relapsed patients with NSCLC SCC other options are “old-fashioned” drugs such as docetaxel, gemcitabine, or ramucirumab plus docetaxel, he said.
In some cases, patients may not even receive treatment. Data from KEYNOTE 407 comparing chemotherapy plus pembrolizumab in first-line SCC and IMPower 131 comparing chemotherapy plus nab-paclitaxel with or without atezolizumab showed that only about 30% of patients received subsequent therapy after relapse and most received chemotherapy.6,7
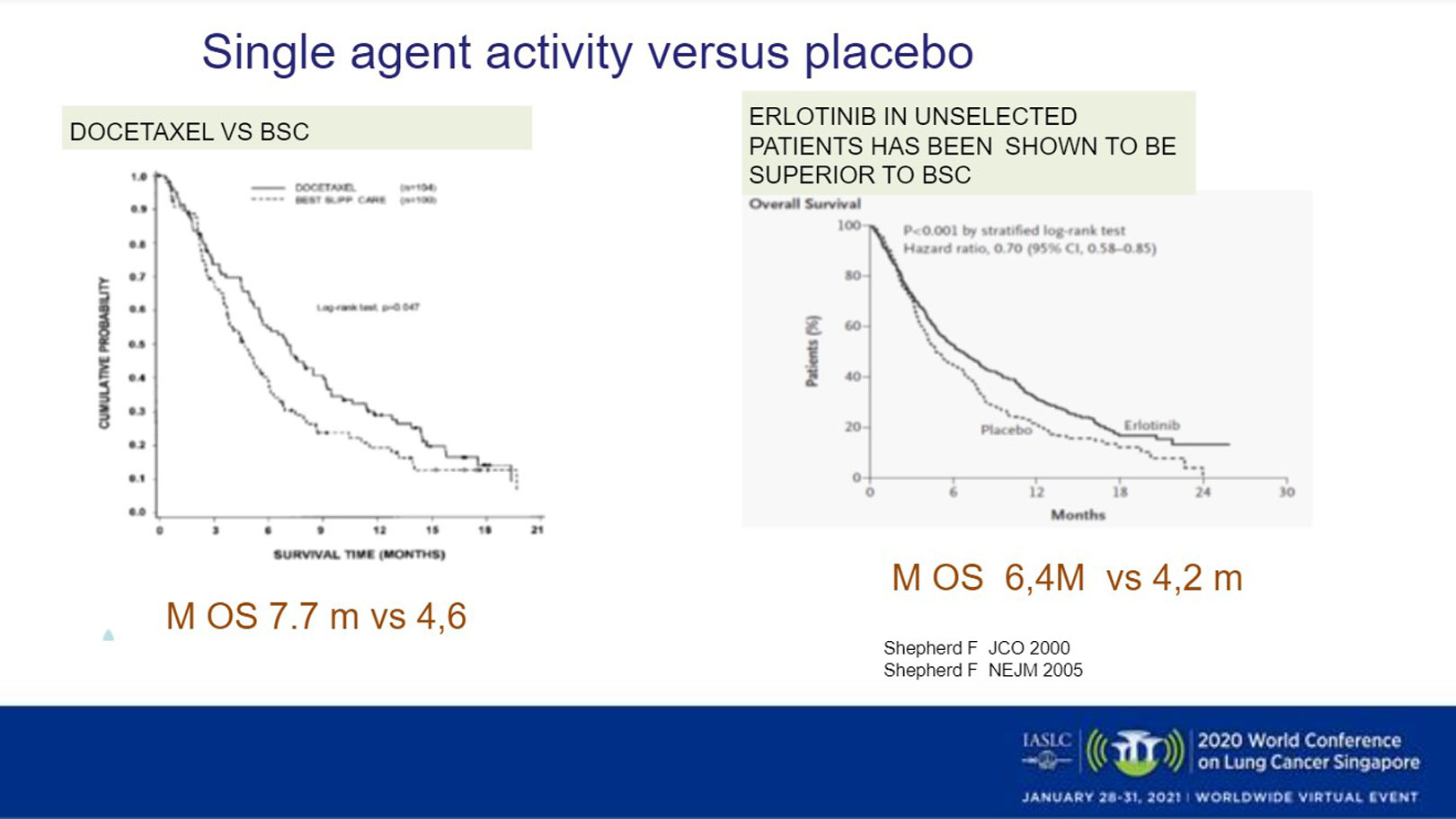
Older trials compare docetaxel against supportive care and show statistically significant benefit with a median overall survival (OS) of 7.7 months favoring docetaxel. Similarly, a trial of erlotinib in unselected patients showed erlotinib to be superior to best supportive care with a modest improvement in OS (Fig 1).8,9 Another trial comparing erlotinib with docetaxel showed a trend toward benefit for docetaxel in the second-line, but in SCC histology activity between the two drugs was similar.10
There is also interest in whether there are any biomarkers indicating use of tyrosine kinase inhibitors (TKIs) instead of chemotherapy. One study looking at the VeriStrat prognostic score showed that patients in the poor prognostic group performed worse with erlotinib and afatinib compared with the good prognostic group, indicating that maybe these patients should not receive TKI inhibitors.12
VEGF may also have a role in resistance to immunotherapy drugs. The phase III REVEL study compared second-line docetaxel with or without ramucirumab. About 25% of participants had SCC histology. There was a modest benefit for PFS and OS for ramucirumab.13 In a subgroup analysis, there was an improved benefit seen in patients with adenocarcinoma histology compared with SCC histology.
However, there is new light on the horizon, Dr. Martin said, with ongoing trials looking at targeting IL1-beta and FGFR1.
For now though, treatment after chemoimmunotherapy represents an unmet need in patients with SCC.
Small Cell
The final presenter was Charu Aggarwal, MD, MPH, of the University of Pennsylvania, Pennsylvania, United States, who gave an update on systemic therapy after chemoimmunotherapy in SCLC.
“Over the last 30 years we have made tremendous progress in application of novel therapies in SCLC,” Dr. Aggarwal said. “Instead of triplet chemotherapies we are able to successfully integrate immunotherapy along with chemotherapy in front-line management.”
The key question now relates to treatment options for second-line. When considering chemotherapy options clinicians must consider which patients will benefit most, when they should use these therapies, and which agent should be used.
When thinking about chemotherapy it is also important to think about the chemo-sensitivity of the disease, she said.
For patients who have relapsed less than 6 months after initiation of first-line therapy there is a whole list of agents that can be considered. Topotecan was the last FDA approved agent prior to the recent approval of lurbinectedin, a synthetic marine derived tetrahydroisoquinoline alkaloid.
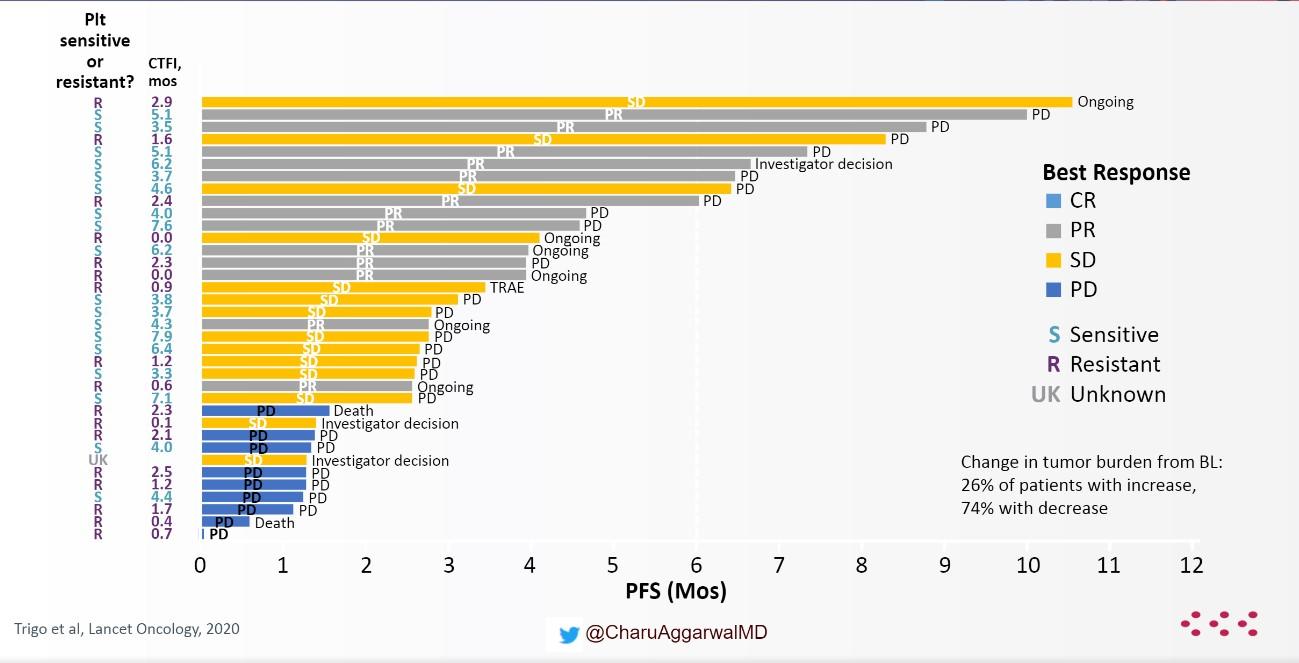
A phase II study evaluated lurbinectedin 3.2 mg/m2 every 3 weeks in 105 patients with SCLC.14 The overall response rate was 35.2%, with a median OS of 9.3 months. When looked the researchers looked at platinum-sensitive and platinum-resistant disease responses were seen in both platinum-sensitive (45%) and platinum-resistant (22%) disease (Fig 2).
“We know that topotecan is an active drug but it is not our favorite regimen because of associated toxicities and myelosuppression,” Dr. Aggarwal said. When looked at in a similar setting, the response rate and median overall survival are higher with lurbinectedin compared with topotecan.
The phase III ATLANTIS trial of second-line lurbinectedin plus doxorubicin compared with physician’s choice of topotecan or cyclophosphamide/doxorubicin/vincristine did not meet the primary endpoint for OS.15 Although, Dr. Aggarwal noted that the dose used in this trial was lower than that used in the phase II trial that led to the drug’s FDA approval.
There are also active investigations of lurbinectedin in combination with chemotherapy, targeted agents, or immunotherapy.
In patients with chemo-sensitive disease, defined as relapse more than 6 months after doublet therapy, the usual practice is to rechallenge with the first-line platinum-based treatment. However, Dr. Aggarwal noted that this approach relies on studies that are more than 20 years old.
In a recent study, 174 patients with sensitive relapsed SCLC were rechallenged with carboplatin plus etoposide versus topotecan.16 The PFS was almost double with carboplatin plus etoposide compared with topotecan. Based on these results, carboplatin plus etoposide represents a reasonable option for use in these patients, Dr. Aggarwal said.
Finally, studies of all checkpoint inhibitors in SCLC in the second-line setting or beyond include studies of nivolumab plus ipilimumab, nivolumab monotherapy, and a pooled analysis of pembrolizumab monotherapy. Both nivolumab and pembrolizumab monotherapies are approved in the third-line and beyond in SCLC; however, these studies were done in patients who had not yet received immune checkpoint inhibitor therapy.
“Immune checkpoint inhibitor monotherapy is not recommended for those patients who progressed after chemoimmunotherapy,” Dr. Aggarwal said.
Finally, despite some hints at possible biomarkers, none has shown consistent predictive potential in patients with SCLC, she said, and none is routinely used in clinical practice at this time.
REFERENCES:
- Park SE, Lee SH, Ahn JS, et al. Increased response rate to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol.2018;13:106-111.
- Merck announces KEYNOTE-598 trial evaluating Keytruda (pembrolizumab) in combination with ipilimumab versus Ketruda monotherapy in certain patients with metastatic non-small cell lung cancer to stop for futility and patients to discontinue. News Release. Merck. November 9, 2020. Accessed November 10, 2020. https://www.merck.com/news/merck-announces-keynote-598-trial-evaluating…. Accessed January 19, 2020.
- Rodriguez-Abreu D, et al. Primary analysis of a randomized, double-blind, phase II study of anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol. 2020;DOI: 10.1200/JCO.2020.38.15_suppl.9503
- Ahn MJ. Vibostolimab, an anti-TIGIT antibody, as monotherapy and in combination with pembrolizumab in anti-PD-1/PD-L1-refractory NSCLC. Annals of Oncology. 2020;31(suppl_4):S754-S840.
- Grohe C, Gleiber W, Haas S, et al. Nintedanib plus docetaxel after progression on immne checkpoint inhibitor therapy: insights from VARGADO, a prospective study in patients with lung adenocarcinoma. Future Oncol. 2019;15:2699-2706.
- Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. Journal of Thoracic Oncology. 202015:1657-1669.
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. Impower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol.2018;DOI: 10.1200/JCO.2018.36.18_suppl.LBA9000.
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus vest supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol.2020;18:2095-2103.
- Shepherd FA, Pereira JR, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med. 2005;353:123-132.
- Garassino M, Martelli O, Broggini M, et al. erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol.2013;14:981-988.
- Soria J-C, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16:897-907.
- Gaadgeel S, Goss G, Soria J-C, et al. Evaluation of the VeriStrat® serum protein test in patients with advanced squamous cell carcinoma of the lung treated with second-line afatinib or erlotinib in the phase III LUX-Lung 8 study. Lung Cancer. 2017;109:101-108.
- Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomized phase 3 trial. Lancet. 2014;384:665-673.
- Trigo J, Subbiah V, Besse B, et al. Lurbinectidin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21:645-654.
- Jazz Pharmaceuticals. Jazz Pharmaceuticals and PharmaMar Announce Results of ATLANTIS Phase 3 Study Evaluating Zepzelca™ in Combination with Doxorubicin for Patients with Small Cell Lung Cancer Following One Prior Platinum-containing Line. December 3, 2020. https://investor.jazzpharma.com/news-releases/news-release-details/jazz… Accessed January 18, 2021.
- Baize N, Monnet I, Greillier L, et al. Carbplatin plus etoposide versus topotecan as second-line treatment for patients with sensitivie relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020;21:1224-1233.