The results of one phase II study looking at PET/CT-guided adaptive radiation therapy and one PET boost trial in locally advanced NSCLC were presented in an Oral Abstract Session (OA2).
Previously published, long-term results of the ROTG 0617 trial showed that high-dose (74 Gy given in 2 Gy daily fractions that prolonged overall treatment time) radiation with concurrent chemotherapy was harmful for patients in terms of safety and efficacy compared with standard dose (60 Gy).1 These two studies – RTOG1106/ACRIN-6697 and PET-Boost – attempted to use more selective approaches to delivering higher radiation using FDG-avid metabolic active tumor volume as guidance either before radiation treatment or mid-treatment.
RTOG1106
Results of the RTOG1106/ACRIN-6697 study showed that biologically adaptive radiotherapy escalation was safe and feasible in a multicenter setting for patients with stage III NSCLC, according to Feng-Ming (Spring) Kong, MD, PhD, Professor of Hong Kong University Shenzhen Hospital and Queen Mary Hospital as well as Case Western Reserve University, who presented the results.
According to Dr. Kong, traditional treatment for lung cancer radiation has been a one-size-fits-all approach; however, tumor, patients, and organs at risk change from week to week during radiotherapy.
In this study, Dr. Kong and colleagues tested if biologic imaging-guided adaptive radiation therapy (BigART) targeting higher doses to resistant aggressive tumor identified on mid-treatment PET-CT, limited by each individual’s tolerance of their normal tissues, could improve local-regional tumor control in patients with stage III NSCLC treated with concurrent chemotherapy.
The study included 138 patients with stage III disease who were medically fit for concurrent chemoradiation. Patients were randomly assigned 1:2 to standard arm of 60 Gy or an adaptive arm. The adaptive arm had pre-radiotherapy planning target volume (PTV) de-escalated to 50 Gy, pre-radiotherapy clinical target volume (CTV) of ≥ 60 Gy, during radiotherapy CT-PTV of ≥70 Gy, and during radiotherapy PET-PTV of up to 80 Gy. All patients had FDG-PET performed around 40 Gy mid-treatment and adaptive radiotherapy escalated during the last nine treatments, with all treatments delivered in 30 daily fractions.
The primary endpoint was 2-year local-regional control rate.
The median prescription dose for the adaptive arm was 71 Gy compared with the 60 Gy standard arm (p < 0.01). The median mean lung dose was 17.9 Gy in the adaptive arm compared with 20.0 Gy of the protocol plan.
There were no significant differences in variations in tumor and organ at risk between the two arms. There were also no significant differences in protocol variations of dose-volume analysis between the two arms. Although Dr. Kong noted that protocol compliance rates were low for both arms. In the overall dose-volume analysis treatment was given per protocol in 50% of patients in the standard treatment arm and 32.4% of patients in the adaptive treatment arm.
There was no significant difference in grade 3 or worse radiotherapy induced lung toxicity (5.3% for standard vs. 6.9% for adaptive), esophagitis (7.9% vs. 3.8%) or heart toxicity (2.6% vs. 1.3%). Grade 2 esophagitis was numerically higher in the adaptive arm (42.5% vs. 31.0%), she said.
The overall 2-year local-regional tumor progression-free time was 27.5 months for standard treatment compared with 28.4 months for the adaptive arm. The site-reported in-field local-regional progression free rate was numerically better for the adaptive arm with an 11% difference at 2 years, corresponding to the median tumor dose difference of 11 Gy. There was a 17% difference in primary tumor control at 2 years, in favor of the BigART arm. In-field tumor control is shown in Figure 1.
“In the future, we believe that optimizing radiation dose to an individual’s radiosensitivity can improve overall survival based on genomic data from RTOG617 presented at ASTRO 2020,” Dr. Kong said. “BigART can increase normal tissue sparing to improve survival on top of dose optimization in each individual.”
PET-Boost
Saskia Cooke, The Netherlands Cancer Institute, Amsterdam, presented results of the phase II, randomized PET-Boost trial (NCT01024829), which showed excellent local control rates in patients with locally advanced NSCLC.
“From previous studies we know high doses of radiation may prevent local failures,” Dr. Cooke said. “However, it is currently unclear if a higher radiation dose should be delivered to the primary tumor as a whole or if a boost redirected to the sub-volume is more effective.”
The PET-Boost trial enrolled 107 patients with stage II-III NSCLC and randomly assigned then to hypofractionated dose escalation to either the primary tumor as a whole (arm A) or high FDG-uptake region inside the primary tumor (>50% SUVmax; Arm B). Concurrent/sequential/no chemotherapy was allowed. Local failure was defined as 20% or more growth of the primary tumor. Regional failure was lymph node failure either in-field or out-of-field on CT-scan.
Results on freedom from local failure at 1 year and overall survival were reported at ESTRO 2020. In central CT review, the freedom from local failure at 1 year was 97% for Arm A and 91% for Arm B.2
The trial was open from April 2010 to September 2017. Due to slow accrual the trial was closed early after inclusion of 150 patients; 107 were randomly assigned. The phase II trial design did not compare arms, so no p values were reported.
Most patients in the study were male (69%), in their late 60s, and had good performance status. Most frequently, patients had stage III disease at diagnosis and were treated with platinum-based concurrent chemotherapy.
The gross tumor volume of the primary tumor was quite large in both arms (100 cm3 for Arm A vs. 115 cm3 for Arm B). Dose per fraction was 3.3 Gy for Arm A and 3.5 Gy for Arm B. The total physical dose was 78 Gy for Arm A compared with 84 Gy for Arm B.
Median follow-up time was 12.6 months. In both treatment arms, the local recurrences at 1 year were low with excellent control, Dr. Cooke said. For Arm A, 12 patients had locoregional recurrence during follow-up. At 2 years, 11% had local recurrence and 28% had regional recurrence. In Arm B, 15 patients had locoregional recurrence. At 2 years, 18% had local recurrence and about 25% had regional recurrences.
The researchers also looked at intrathoracic site of first recurrence (Fig 2). In Arm A there were 18 intrathoracic recurrences. Three patients had local failure. There were eight regional in-field failures, six regional out-of-field failures, and eight new pulmonary lesions. In Arm B, there were 19 intrathoracic recurrences. There were four regional in-field failures, six regional out-of-field failures, four local failures, and seven new pulmonary lesions.
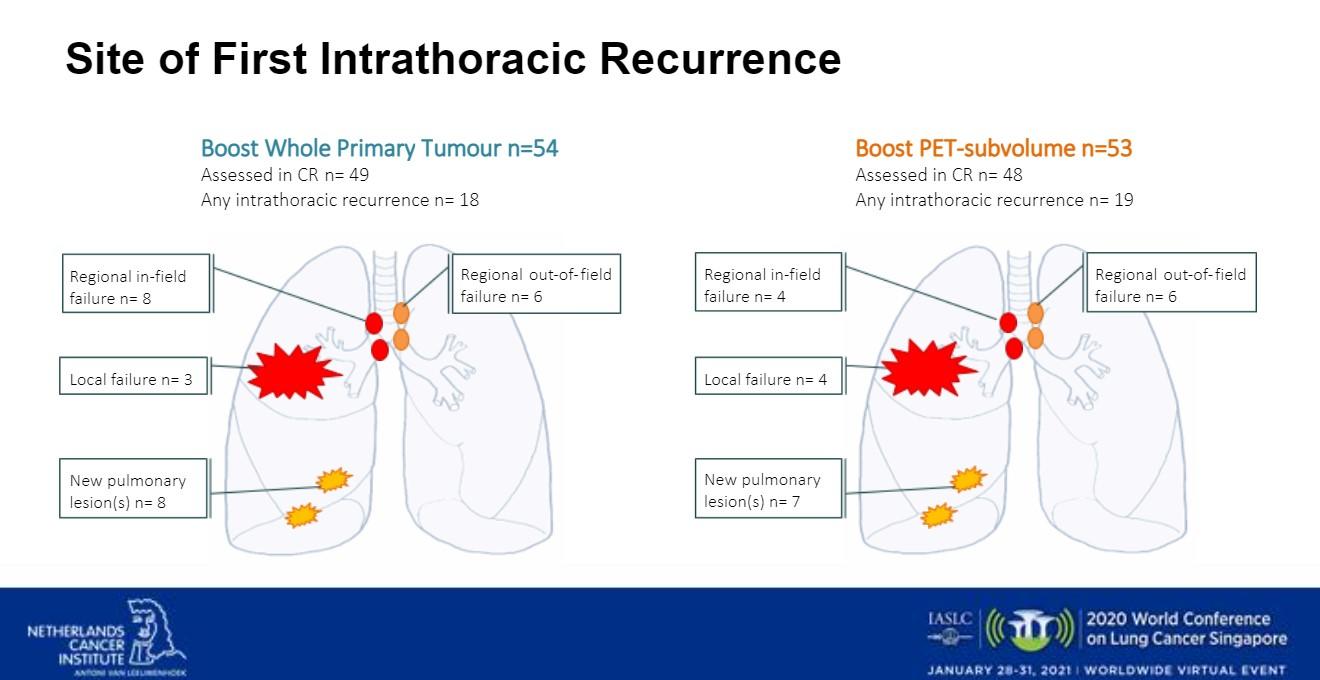
Dr. Cooke noted that analysis of the study’s results are still ongoing. As a take-home message, she said that the researchers think that in selected patients with locally advanced NSCLC using hypofractionated dose escalation to the primary tumor is an important subject for future research.
You Lu, MD, of West China Hospital Sichuan University, was the discussant of these two abstracts on PET/CT-guided boost radiation therapy testing high FDG-uptake regions as a predictive marker.
“There was similar background and hypotheses of these two studies,” Dr. Lu said. “[They tested] the idea that both pre-radiotherapy and post-radiotherapy primary tumor SUVmax of PET/CT can predict the outcome of radiotherapy in patients with NSCLC.”
In RTOG 1106, the researchers did not find a significant benefit from adaptive radiotherapy on local-regional control, compared with control arm (p =0.6585), Dr. Lu said. Furthermore, there was no significant benefit from adaptive radiotherapy for progression-free survival and overall survival. Although, he noted, there was acceptable adverse events in the adaptive radiotherapy arm.
For PET-Boost, Dr. Lu said, there can be no definite conclusions because no p values were reported. Additionally, slow accrual leading to trial closure limited the number of patients studied.
“Increasing the in-field radiation dose is important, but is not the only factor involved,” he said. In theory, radiotherapy with higher the biological effective dose could bring about better the local-control. However, in clinical practice, it is difficult to get an ideal tumor local control just by increasing dose in patients with LA-NSCLC, because of radiation tolerance of normal organ at risk.
RTOG 0617 was not targeting the high FDG-uptake region, but this trial had a negative conclusion when testing the higher 74 Gy dose with the standard 60 Gy dose. The results showed that the higher dose was harmful with concurrent chemoradiotherapy for patients with locally advanced NSCLC.
As researchers pursue their aim to increase the cure rate on locally advanced disease, they should consider other biological predictive indicators, such as PD-L1. In the PACIFIC study, there was improved local control and overall survival with immune checkpoint inhibitor consolidation.3,4 At the 3-year survival update the difference in survival with durvalumab compared with placebo was 8% at 1 year, 11% at 2 years, and 13.5% at 3 years with greater benefit seen in patients with higher PD-L1 expression.
Beyond high FDG-uptake by PET and expression of PD-L1, circulating tumor DNA dynamics may predict benefit from consolidation immunotherapy in locally advanced disease, Dr. Lu said.
References:
- Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG Oncology RTOG 0617: standard-versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol.2020;38:706-714.
- Lalezari F, Lambrecht M, Lewensohn R, et al. The PET-boos trial: isotoxic homogeneous or FDG-directed dose escalation in stage II-III NSCLC. Abstract OC-0609. Presented at ESTRO; November 30, 2020.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. N Engl J Med. 2017;377:1919-1929.
- Gray JE, Villegas A, Daniel D, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC—update from PACIFIC. Journal of Thoracic Oncology. 2019;doi:10.1016/j/jtho.2019.10.002.
- This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 60 days at wclc2020.iaslc.org.










