While checkpoint inhibitors have improved the outlook for patients with advanced NSCLC, more information is needed about the long-term toxicities caused by the drugs and how they should be managed.
A retrospective study (MA07.05) shed light not only on the power of PD-1/PD-L1 inhibitors to improve overall survival (OS) in this population but also on the duration and treatment of the immune-related adverse events (irAEs) that often accompany their use.
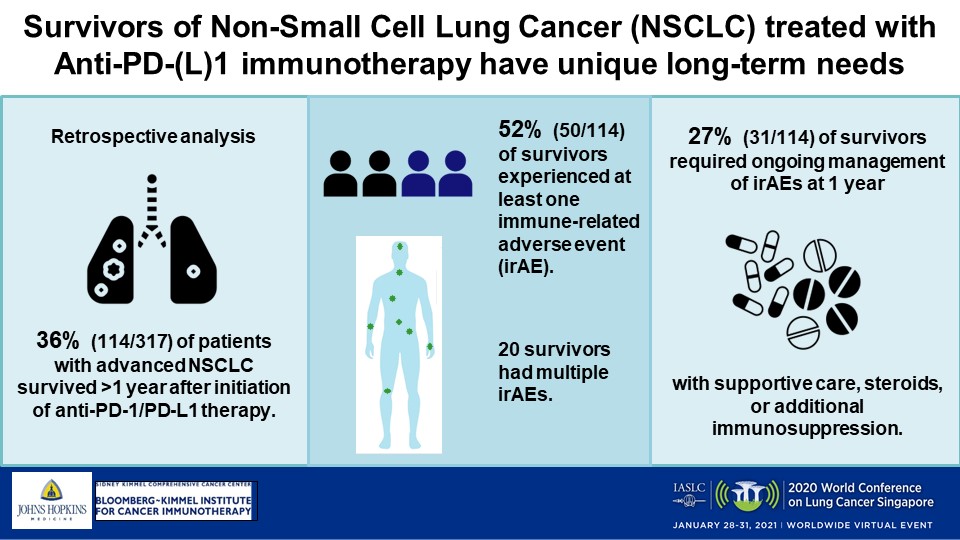
In the small study of a real-world cohort, Melinda Hsu, MD, and colleagues reported that 36% of patients lived more than 1 year after therapy’s start, with nearly 31% of those survivors experiencing irAEs that persisted beyond 12 months, requiring continuous treatment.
“We believe these data, in combination with an ongoing interview study we are conducting in survivors of NSCLC treated with immunotherapy, could be used to develop a survivorship program for patients treated with immunotherapy focused on long-term toxicity management,” Dr. Hsu, an oncology fellow at Johns Hopkins Medicine, said.
Study Findings
The researchers selected patients from a Johns Hopkins database, analyzing the chart information of 317 with stage III or IV disease who were treated with immunotherapy between November 2009 and February 2020. They found that 36% (114) had lived longer than a year on the therapy. Most of those long-term survivors were male (54%) and a median of 66 years old (range: 42 to 87). A total of 82% (94) were people who previously or currently smoked, 67% (76) had adenocarcinoma, 34% (32) had tumors that harbored KRAS mutations, and 61% (69) had an undetermined PD-L1 status, Dr. Hsu said.
Of the 114 long-term survivors, she said, 52% (59) had received standard-of-care immunotherapy outside a clinical trial and 82% (94) had received checkpoint inhibition in the first- or second-line setting. Most frequently administered were nivolumab and pembrolizumab (39% and 18% of the cohort, respectively). Some patients received combination regimens: 13% an anti-PD-1/PD-L1 immunotherapy plus another type of agent, 11% nivolumab plus ipilimumab, and another 11% a checkpoint inhibitor along with chemotherapy, the researchers wrote in an abstract. The median number of immunotherapy doses received was 13 (range: 1-121).
Dr. Hsu’s team found that median OS, defined as the time from immunotherapy’s start until death from any cause, was an “unprecedented” 26.5 months (range: 12.2-106.9) and that median progression-free survival (PFS) was 13 months (range: 0.7-106.9). For 85% of participants, the best response was a partial one or stable disease.
IrAEs of any grade were experienced by 59 of the survivors (52%), with 20 having more than 1 event and 3 patients developing up to 5. Most common were pneumonitis (18%), dermatitis (11%), and inflammatory arthritis (11%). The majority of the irAEs were CTCAE grades 1 or 2 (80%), while less than one-quarter of affected patients experienced grade 3 events and 1 individual had a grade 4 irAE, Dr. Hsu said.
Across the 59 who developed irAEs, the median time until first onset was 15 weeks. For those who developed multiple irAEs, first onset was at 9 weeks, compared with 22 weeks for patients who developed a single irAE (P=0.03), Dr. Hsu said. Fifty-one percent of those with irAEs (30 of 59) were treated with steroids alone, while 12% (7) required additional immunosuppression, the researchers wrote. Adverse events led 29 survivors, or 25%, to discontinue immunotherapy.
“Most significantly,” of the 59 survivors who experienced irAEs, 31 (27%) had ongoing symptoms at death or last follow-up, Dr. Hsu said. Fourteen were being treated with steroids at that point and 5 were receiving additional immunosuppression.
The researchers wrote that the development of an irAE was associated with improved PFS: Those who reported toxicities experienced disease worsening at a median 21.2 months compared with a median 9.8 months for patients without toxicities (P=0.002). That pattern did not hold true when it came to OS, with patients who experienced irAEs logging a median survival of 28.5 months versus 24.5 months for those who did not encounter any events (P=0.28).
Next Steps
Ivy Elkins of Illinois, a survivor of metastatic, EGFR-positive stage IV lung cancer and a co-founder of the patient advocacy group EGFR Resisters, called the study an important investigation into an issue that is not widely understood.
“These toxicities are significant and can be managed, but awareness about this issue is important for setting expectations for docs and patients,” she said.
The study suggested to discussant Haryana Dhillon, PhD, of the University of Sydney, that “we need to be better at monitoring and to try and identify better predictors of immunotherapy-related adverse events.” She appreciated the study’s real-world population but said researchers should consider conducting prospective interventions.
“We are clearly seeing that lung cancer survivorship is very important, and it really needs a coordinated international effort to try and improve the experiences for our patients,” Dr. Dhillon said.