Curative therapy remains elusive in early-stage NSCLC following surgical resection, sometimes even for patients with stage I disease. Adjuvant therapy can be of benefit, but only for a select few. As shown in the Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis, platinum doublet chemotherapy, the current standard of care following complete resection of stage II/III NSCLC, only improved 5-year survival by 5.4%.1
“There is clearly unmet need,” stressed Tetsuya Mitsudomi, MD, PhD, of Kindai University in Japan. He believes the field can begin to address this unmet need for patients with resectable disease by leveraging liquid biopsy techniques to detect the presence of minimal residual disease (MRD)—the malignant cells that remain despite a complete response to treatment and that share phenotypic and genetic heritage with the original tumor cells.
“Detection of MRD or micrometastatic tumor cells in a post-operative patient has long been a dream for us surgeons,” emphasized Dr. Mitsudomi.
As he explained, knowing which patients will not be cured by surgery alone allows these individuals to be singled out for adjuvant therapy while sparing all others from unnecessary treatment. “Although many prognostic factors, such as mutations of the KRAS or P53 genes, have been proposed [to identify individuals who need adjuvant therapy], most of them were not potent or reproducible enough to be used in routine clinical practice. However, the detection of MRD appears to be a very reliable prognostic marker,” Dr. Mitsudomi said.
The most common method used to detect MRD involves circulating tumor DNA (ctDNA) testing. ctDNA detection has typically been more successful in patients with metastatic disease as compared with patients who have recently undergone resection. “The detection of ctDNA in surgical patients has been a challenge because of lower amounts. However, the recent advent of next-generation sequencing technology and enrichment technology makes it possible to raise the sensitivity as well as the specificity,” according to Dr. Mitsudomi.
The Way Forward
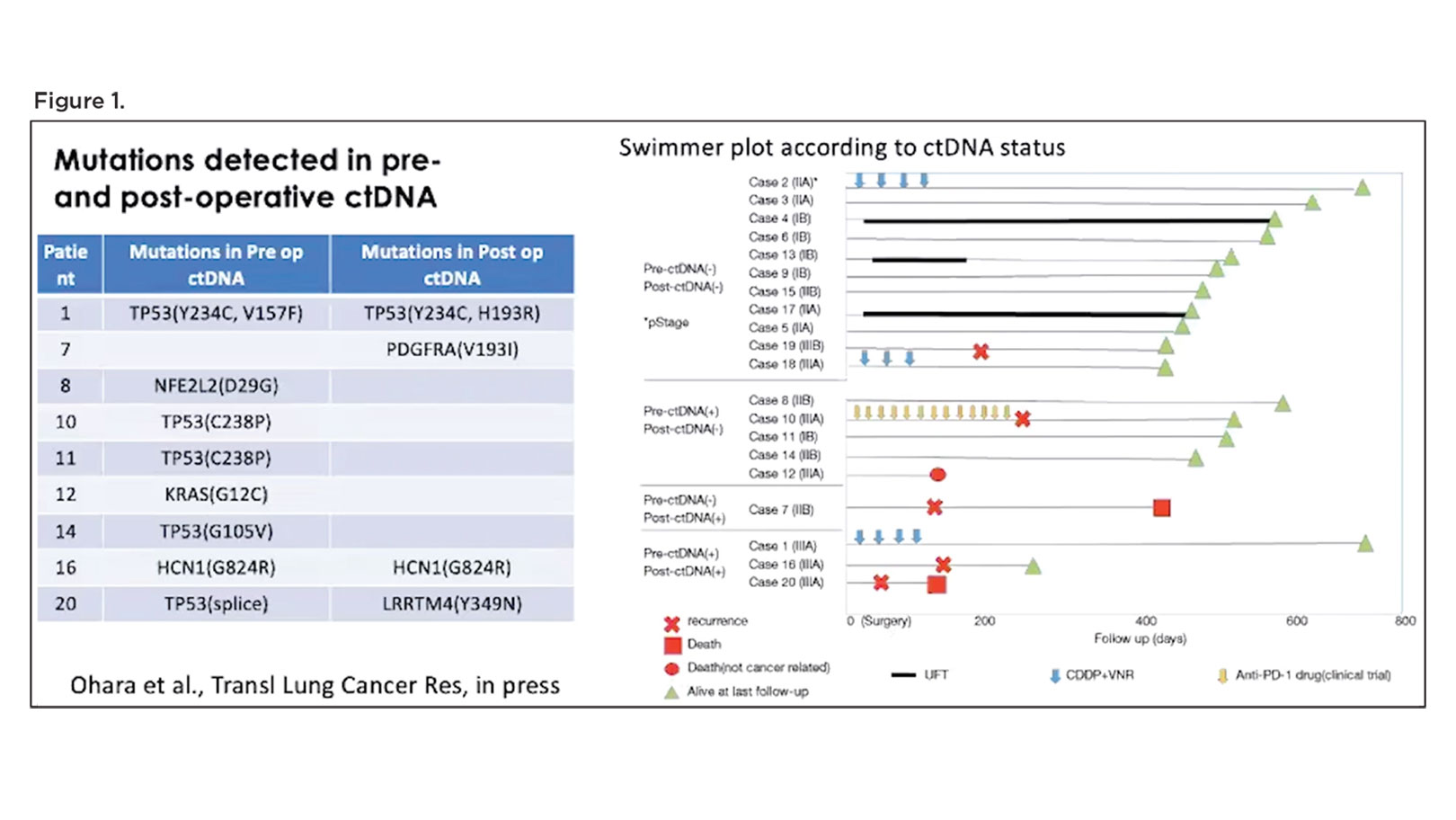
Putting these technologies to use, a handful of studies in lung cancer and other malignancies have emerged illustrating the power of ctDNA detection in heralding disease progression following curative therapy, well before disease becomes radiographically apparent. Building on this work, Dr. Mitsudomi and his colleagues recently performed a pilot study in 20 patients with stage IIA through IIIA NSCLC in which they used cancer personalized profiling by deep sequencing (CAPP-Seq) to detect the presence of ctDNA in plasma samples collected before surgery and then 3 to 12 days after surgery.2 Patients who had undetectable ctDNA following resection demonstrated the best outcomes with regard absence of disease and survival (Figure 1). In contrast, patients with detectable ctDNA after surgery showed a higher likelihood of disease recurrence and death.
“Although the number of the patients is small, we think that we were able to confirm the feasibility of ctDNA analysis, as well as the prognostic significance of the ctDNA in our patients,” remarked Dr. Mitsudomi.
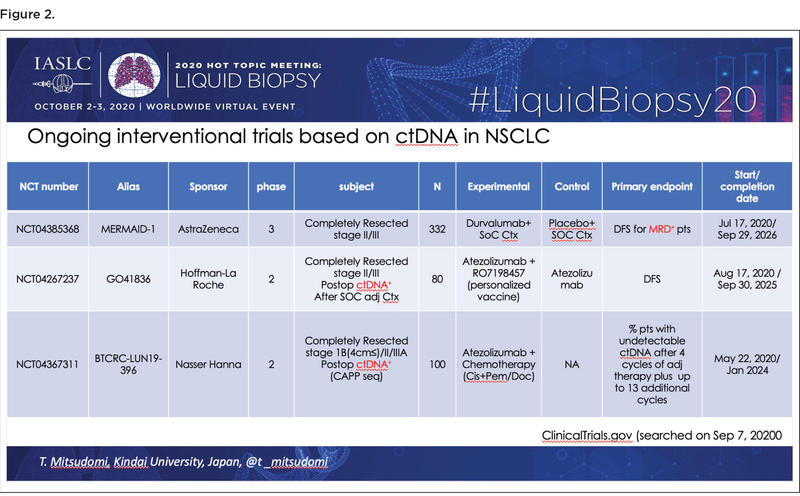
Since MRD detection with ctDNA appears to be a strong and reproducible prognostic factor based on research to date, the next logical step is to determine whether administering adjuvant treatment to patients who have MRD following curative therapy can improve outcomes. Dr. Mitsudomi identified three ongoing interventional trials addressing this issue by analyzing the value of intensified adjuvant therapy among patients with NSCLC who are ctDNA-positive following surgical resection (Figure 2).
Ultimately, Dr. Mitsudomi hopes that stratification of patients by ctDNA analysis following surgery will enable clinicians to better determine exactly which individuals need adjuvant therapy. Th e goal would be to deliver adjuvant treatment to patients with MRDpositive disease given their higher risk for recurrence, while sparing individuals with MRD-negative disease from further treatment that is likely to add only an incremental benefit, if any at all.
References
1. Pignon JP, Tribodet H, Scagliotti GV, et al; LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552-3559.
2. Ohara S, Nishio K, Mitsudomi T, et al. Prognostic implications of pre- versus post-operative ctDNA in surgically resected lung cancer patients: A pilot study. Trans Lung Cancer Res. In press.