Lung cancer screening saves lives. This once controversial statement has now been validated in recent large, randomized trials of low-dose computed tomographic (LDCT) lung cancer screening (LCS) including the National Lung Screening Trial (NLST)1 and the Dutch-Belgian Lung Cancer Screening Trial.2 However, widespread implementation of LCS has been limited. In the United States, screening rates are dismal, ranging from 3% to 15% of all eligible patients by U.S. Preventive Services Task Force criteria. Reasons for this poor penetrance of LCS include concerns for overdiagnosis and a perceived high rate of iatrogenic harm from unnecessary interventions. Unfortunately, the lack of lung cancer screening represents a significant lost opportunity to save lives. It has been estimated that 51.6% of lung cancer deaths could be prevented if all eligible patients received appropriate LCS.3 Our study “Surgery and invasive diagnostic procedures for benign disease are rare in a large low-dose computed tomographic lung cancer screening program,” which was presented in May 2020 at the 100th Annual Meeting of the American Association for Thoracic Surgery and published in The Journal of Thoracic and Cardiovascular Surgery, evaluated the risk of unnecessary intervention in the context of a contemporary LCS program and multidisciplinary thoracic oncology practice.4
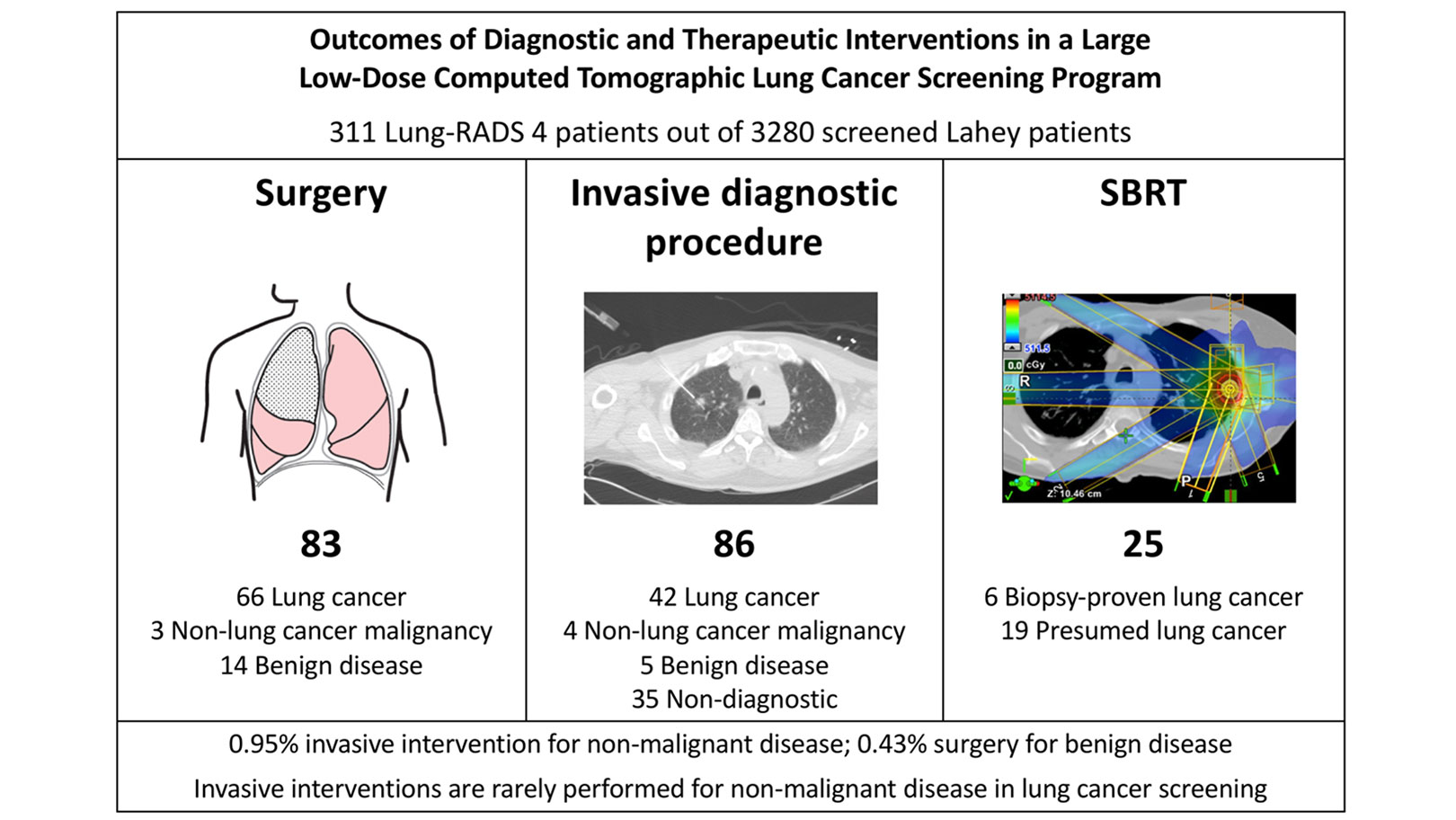
The Lahey Hospital and Medical Center (formerly Lahey Clinic) in Burlington, Massachusetts, has performed more than 20,000 LDCT lung cancer screening studies since the inception of the program in 2012. This study evaluated a total of 3,280 consecutive patients from our institution between January 1, 2012, and June 30, 2017, with clinical follow-up through January 2019. A total of 311 patients with complete follow-up had concerning LDCT findings (Lung CT Screening Reporting and Data System [Lung-RADS® 4]). These patients were evaluated for rates of diagnostic or therapeutic interventions, pathologic diagnoses, complications, and outcomes. The breakdown of patients is illustrated in the Figure.4
A total of 93 patients (29.9% of the Lung-RADS 4 patients) were diagnosed with lung cancer. Eighty-three patients underwent lung surgery (2.5% of screened patients). Fourteen patients underwent surgery for benign disease (0.43% of screened patients). An additional 54 patients, 5 of whom were diagnosed with benign disease, underwent at least one invasive diagnostic procedure, but not surgical resection. Therefore, the incidence for any invasive procedure for benign disease was 0.95%. Twenty-five patients (0.76%) underwent stereotactic body radiotherapy. In addition, we demonstrated no differences in the rates of surgical resection or any invasive intervention for benign disease between Group 1 and 2 National Comprehensive Cancer Network high-risk categories.
Similar to the experience in the NLST trial, we have seen an increase in diagnosis of early-stage lung cancer after implementation of LCS. In 2017, 86.1% of lung cancers diagnosed in our LCS program were stage I. This shift toward earlier-stage disease has promoted more frequent utilization of minimally invasive surgery and lung-preserving approaches for patients, when appropriate. In our series, 90.4% of lung resections were performed with a minimally invasive approach, and sublobar resection was utilized in 48.2% of cases. There were no deaths within 60 days of any invasive diagnostic procedure or surgery. Five patients who underwent surgery (6.0%) had a “major” complication according to the criteria as defined in the NLST compared to a reported rate of 11.9% in the NLST trial. Of the 54 patients undergoing an invasive procedure, but not surgical resection, three developed post-procedure pneumothorax requiring hospital admission with a median 3-day length of stay, and there were no other complication-related hospital admissions.
Within the context of this retrospective study’s limitations, we demonstrated that invasive procedures are rarely performed for non-malignant disease in LCS (0.95% incidence in the Lahey program). Similarly, morbidity from unnecessary intervention is exceedingly rare. Multi-disciplinary oversight, use of the Lung-RADS structured reporting system, and continuous monitoring of outcomes ensures the safety of LCS. The results reported in our study should allay concerns regarding unnecessary diagnostic interventions or surgery and the misperception of iatrogenic harm that continue to hinder adoption of LCS. Furthermore, it is important to note that surgery for benign disease, while important to minimize, is often not “unnecessary,” as it provides a definitive diagnosis that may be actionable even in the absence of malignancy. Overall, the benefits of LDCT lung cancer screening appear to far outweigh the small associated risks. Wider adoption of LCS will lead to diagnosis of earlier-stage disease, increased opportunity for cure, and, ultimately, fewer lives lost from lung cancer.
References:
- National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503-513.
- Cheung LC, Katki HA, Chaturvedi AK, Jemal A, Berg CD. Preventing Lung Cancer Mortality by Computed Tomography Screening: The Effect of Risk-Based Versus U.S. Preventive Services Task Force Eligibility Criteria, 2005-2015. Ann Intern Med. 2018;168(3):229-232.
- Ho H, Williamson C, Regis SM, et al. Surgery and invasive diagnostic procedures for benign disease are rare in a large low-dose computed tomography lung cancer screening program. J Thorac Cardiovasc Surg. 2020 Sep 8:S0022-5223(20)32573-3. (Online ahead of print).