
Research presented during IASLC’s 2022 World Conference on Lung Cancer supports the continued investigation of pembrolizumab-based combinations for patients with small cell lung cancer (SCLC).
Charles M. Rudin, MD, PhD, chief of the thoracic oncology service and co-director of the Druckenmiller Center for Lung Cancer Research at Memorial Sloan Kettering Cancer Center, New York, shared long-term follow-up results (abstract 1849) from the phase 3 KEYNOTE-604 study of pembrolizumab plus etoposide/platinum (EP) compared to placebo plus EP as first-line therapy for extensive stage SCLC.
“Pembrolizumab and EP continued to show clinically meaningful improvement in survival and manageable safety versus placebo and EP in patients with previously untreated extensive stage SCLC,” Dr. Rudin said. “The 3-year overall survival rate was more than two-and-a-half times higher among patients who received pembrolizumab and EP. Patients who completed 35 cycles of pembrolizumab had durable responses.”
Previous KEYNOTE-604 data showed progression-free survival (PFS) was significantly improved with pembrolizumab plus EP versus placebo plus EP (HR, 0.75 [95% CI, 0.61–0.91]; P=0.0023). And although the hazard ratio for overall survival (OS) favored pembrolizumab plus EP in the study, the significance threshold was not met (HR, 0.80 [95% CI, 0.64–0.98]; P=0.0164).
Building on this earlier data, Dr. Rudin presented updated results with a median of 3.5 years of follow-up in patients who completed the maximum of 35 cycles of pembrolizumab.
In KEYNOTE-604, eligible patients with previously untreated extensive stage SCLC were randomized 1:1 to pembrolizumab 200 mg or placebo for up to 35 cycles, plus four cycles of standard-dose EP. Dual primary endpoints were OS and PFS in the intention-to-treat (ITT) population.
Of the 453 randomized patients in the ITT population (pembrolizumab + EP, n=228; placebo + EP, n=225), median time from randomization to data cutoff (September 21, 2021) was 43.3 (37.8 to 52.3) months. In the pembrolizumab + EP group, 54.8% of patients received subsequent therapy and 66.2% of patents in the placebo + EP group received subsequent therapy (11.2% vs. 22.1% received subsequent immune checkpoint inhibition, respectively).
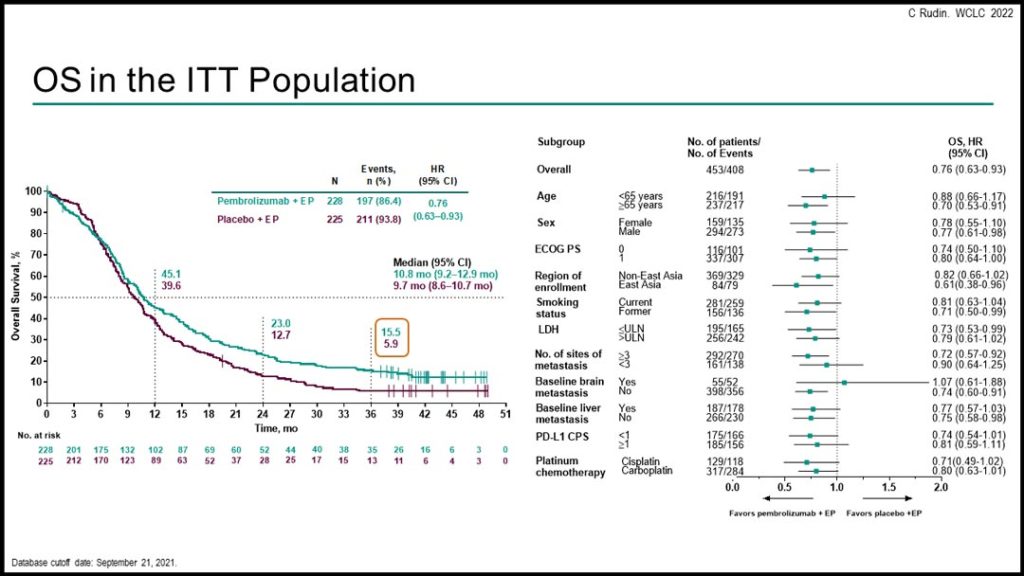
Efficacy outcomes, including OS and PFS, were improved with the pembrolizumab + EP combination. Three-year OS was 15.5% among patients treated with pembrolizumab + EP versus 5.9% in those treated with placebo + EP. Grade 3-5 adverse events occurred in 78.9% of patients in the pembrolizumab + EP group, and in 77.1% of patients in the placebo + EP group.
Eighteen patients completed 35 cycles of pembrolizumab. Of these patients, 14 were alive as of the last assessment before data cutoff. Overall response rate among these patients was 100% (95% CI, 81.5%–100%; 2 CR, 16 PR), and median duration of response was not reached (14.1 to 46.8-plus months). From the time of completing 35 cycles (~two years), median OS was not reached (95% CI, 16.6 months to not reached). Two-year OS rate (95% CI) from the time of completing 35 cycles of pembrolizumab was 72.2% (39.5%–89.2%).
Dr. Rudin said he believes the follow-up data support the continued exploration of pembrolizumab-based combinations for patients with small cell lung cancer.





