Immunotherapy with PD(L)-1-directed immune checkpoint inhibitors, alone or in combination with chemotherapy, has been the cornerstone in upfront treatment of PD-L1–expressing advanced non-small cell lung cancer (aNSCLC) without oncogenic driver mutations for nearly a decade. The HARMONi-2 phase III study demonstrated significantly longer median progression-free survival (PFS) with upfront ivonescimab over pembrolizumab.
John V. Heymach, MD, PhD, Chair of Thoracic/Head and Neck Medical Oncology at MD Anderson Cancer Center, discussed the findings during the first of two Presidential Symposia taking place during the 2024 World Conference on Lung Cancer. IASLC President-elect Caicun Zhou, PhD, MD, Professor and Director, Department of Medical Oncology at Shanghai Pulmonary Hospital, Tongji University, presented results from the HAROMNi-2 trial.
In HARMONi-2, treatment-naive patients with stage IIB-IV PD-L1–positive (tumor proportion score [TPS] ≥1%) aNSCLC without EGFR mutations or ALKrearrangements were randomized 1:1 to receive either ivonescimab or pembrolizumab, until unacceptable toxicity, no clinical benefit, or up to 24 months. The primary endpoint was PFS by blinded independent central review and secondary endpoints included overall survival, investigator-assessed PFS, response, and safety. Treatment was stopped in the face of disease progression or unacceptable toxicity or continued for up to 24 months.

Dr. Zhou summarized the key findings from a planned interim analysis of PFS. Overall, baseline characteristics of patients in the ivonescimab and pembrolizumab arms were well-balanced. Of the 198 patients in the ivonescimab arm, 54.5% had nonsquamous histology, 12.6% had liver metastases, and 16.7% had brain metastases. PD-L1 TPS was 1%–49% in 42%; the remainder had high PD-L1 (TPS ≥50%).
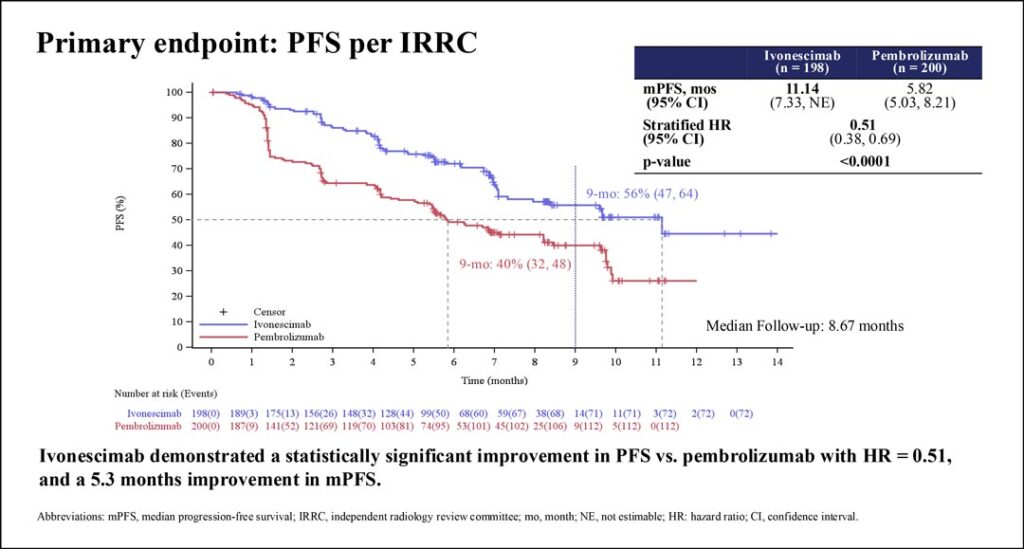
“Median PFS was 11.1 months in ivonescimab compared to 5.8 months in pembrolizumab group with hazard ratio of 0.51,” Dr. Zhou said as the audience applauded.
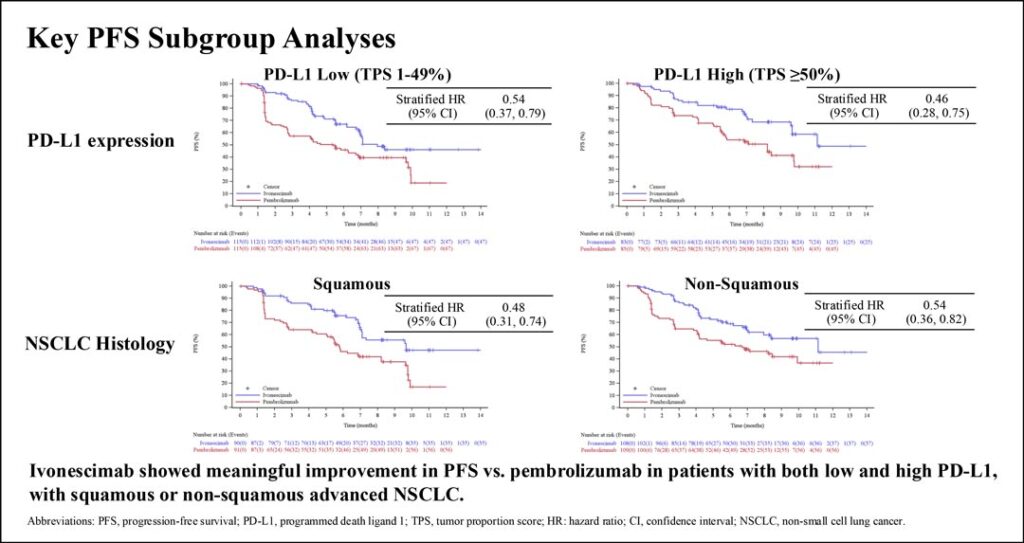
The PFS improvement with ivonescimab was seen in all patient subgroups, including those with either low or high PD-L1 and either squamous or nonsquamous histology. Secondary response endpoints of overall response rates and disease control rates were also higher with ivonescimab than with pembrolizumab. Safety analysis showed that ivonescimab and pembrolizumab had similar immune-related toxicity profiles.
Ivonescimab is a novel first-in-class bispecific antibody that binds both PD-1 and VEGF. This dual targeting mechanism of action allows simultaneous inhibition of PD-1–mediated immune suppression and tumor angiogenesis. Clinical findings showed no unexpected VEGF inhibitor-related toxicities.
In his analysis, Dr. Heymach discussed how concomitant VEGF blockade could enhance the anti-tumor effects of PD-(L)1 inhibition.
“We know that VEGF plays a role in tumor angiogenesis, driving tumor invasiveness,” he said. “It may be less appreciated the role [VEGF] plays in the immune system.”
He outlined how VEGF suppresses anti-tumor immunity via multiple mechanisms and differentiated the role of VEGF receptors 1 and 2—while the former is primary driver of angiogenesis, the latter is more involved in immune modulation.
Previous studies that combined VEGF inhibitors with PD-(L)1 inhibitors have shown a benefit in patients with NSCLC, albeit not as striking as that seen in HARMONi-2, Dr. Heymach said. He also indicated that pembrolizumab monotherapy would not be the preferred comparator arm in the low PD-L1 subgroup. Also, the findings need to be confirmed in a broader population, as HARMONi-2 only enrolled patients in China.
“This study highlights the potential of combined VEGF and PD-(L)1 blockade…” Dr. Heymach said. “If these benefits are confirmed, it is possible that our therapeutic ‘harmony’ in the first-line NSCLC space may come to an end and that is a ‘disharmony’ that we should all be up for.”