Six-year data from the CheckMate 227 trial showed superior survival and health-related quality of life (HRQoL) for first-line nivolumab (nivo) plus ipilimumab (ipi) in patients with metastatic non-small cell lung cancer (NSCLC). Nivo+ipi was superior to chemotherapy for patients with PD-L1 levels ≥1% as well as those with PD-L1 levels <1%.

“This is the longest ever reporting on immunotherapy in metastatic NSCLC with a minimum of 6 years of follow-up for all patients,” said Solange Peters, MD, PhD, Professor and Chair of Medical Oncology and the thoracic malignancies program at Lausanne University Hospital, Lausanne, Switzerland. “There were statistically and clinically important benefits from immunotherapy 4 years after stopping treatment.”
Dr. Peters presented the data on Monday, September 11, during an oral abstract session at the 2023 World Conference on Lung Cancer in Singapore. The session, Immune Checkpoint Therapy: Long Term Follow Up, can be viewed on-demand by registered attendees through December 31.
Her presentation updates 5-year survival data presented earlier in 2023 and provided the first analyses of overall survival by response/tumor burden reduction and baseline HRQoL. Nivo + ipi is already approved for the treatment of metastatic NSCLC in many countries, although approvals require PD-L1 expression ≥1% in some jurisdictions.
CheckMate 227 Part 1A which randomized 1,189 patients with stage IV or recurrent NSCLS who had no prior systemic therapy and no known EGFR or ALK mutations to nivo + ipi (396 patients), chemotherapy (397 patients), or nivo alone (396 patients). Part 1B randomized 550 patients in a similar pattern to nivo + ipi (187), chemotherapy (186), or nivo + chemotherapy (177). The minimum follow-up was 73.5 months, median follow-up was 78.8 months.
In patients with tumor PD-L1 ≥1%, median overall survival (OS) was 17.1 months for nivo + ipi (HR=0.78), 15.7 months for nivo monotherapy (HR=0.91), and 14.9 months for chemotherapy alone. For patients with PD-L1 <1%, median OS was 17.4 months for nivo + ipi (HR=0.65), 15.2 months for nivo + chemotherapy (HR=0.79), and 12.2 months for chemotherapy alone. Six-year progression-free survival (PFS) rates for patients with PD-L1 ≥1% were 11% for nivo + ipi versus 2% for chemotherapy. For patients with PD-L1 <1%, 6-year PFS rates were 8% for nivo + ipi with no patients at risk in the chemotherapy arm.
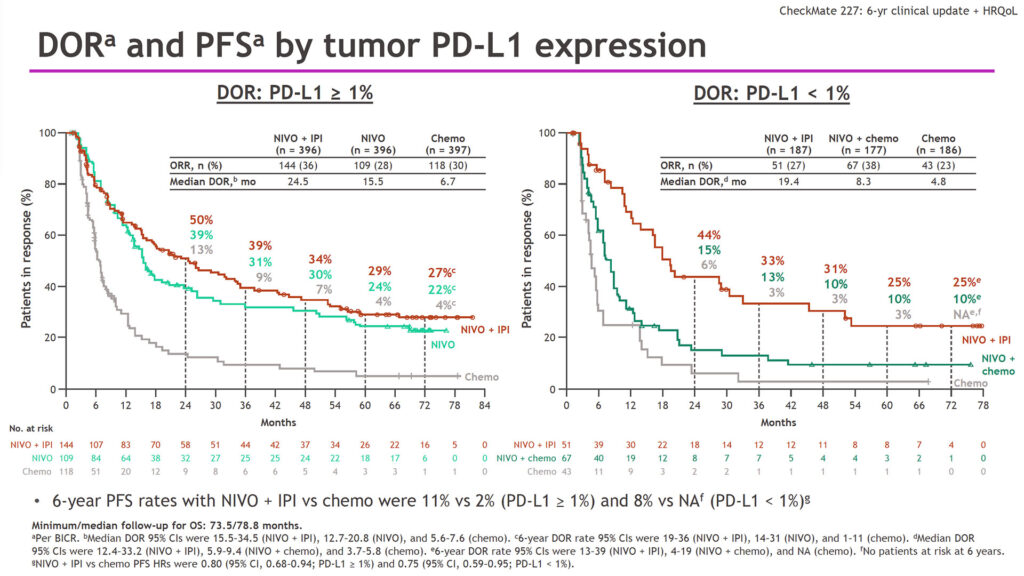
Duration of response (DOR) showed similar patterns. In patients with PD-L1 ≥1%, median DOR was 24.5 months for nivo + ipi, 15.5 months for nivo monotherapy, and 6.7 months for chemotherapy. In patients with PD-L1 <1%, median DOR was 19.4 months for nivo + ipi, 8.3 months for nivo + chemotherapy, and 4.8 months for chemotherapy alone.
OS was associated with improved tumor burden reduction. In patients with PD-L1 ≥1% taking nivo + ipi, 12% showed a partial response of 30% to 50% reduction in tumor burden; 16% a partial response of 50% to 80% reduction in tumor burden; and 8% a partial/complete response of ≥80% reduction in tumor burden. OS at 6 years was 43%, 34%, and 77% respectively.
For patients on chemotherapy alone, 17% had a partial response of 30% to 50% reduction in tumor burden, 15% a partial response of 50% to 80% reduction in tumor burden, and 0 % a partial/complete response of ≥80% reduction in tumor burden. OS at 6 years was 16%, 10%, and 0% respectively.
Higher baseline HRQ0oL scores were positively associated with OS. For patients on nivo + ipi, patients with high baseline HRQoL, median OS was 19.7 months versus 14.9 months for low baseline scores (HR=0.69). In the chemotherapy arm, median OS for high baseline scores was 17.8 months versus 10.1 months for low baseline scores (HR=0.61).
“Nivolumab plus ipilimumab continues to provide long-term, durable benefits versus chemotherapy for patients regardless of PD-L1 level,” Dr. Peters said. “Not surprising, patients with higher tumor burden reduction had greater long-term benefits, and better baseline HRQoL was associated with better overall survival.”





