Since December 2019, the world has been mired in a pandemic that has displaced other 21st-century health priorities for many populations. The social significance and speed of contagion from COVID-19 have altered criteria for public health prioritization, to the detriment of other health problems including noncommunicable diseases, which are recognized as the leading cause of preventable illness and premature death. According to the World Health Organization, noncommunicable diseases are responsible for three out of every four deaths. Cancer is the second most frequent after cardiovascular diseases.1
Likewise, in Latin America and the Caribbean, cancer ranks second as a cause of death, with 672,758 deaths recorded in 20181
. Furthermore, during 2018 the incidence of new cases rose to 1,412,732 events, explained by the region’s aging population, exposure to risk factors, changes in lifestyle, and progressive economic development (transition from acute infectious diseases to chronic pathologies, with particular emphasis on cancer and diabetes).2
Globally and regionally, cancer is one of the leading health challenges. In addition to its importance as a cause of death and suffering, the cost of cancer also has widespread impact.3
COVID-19 and Cancer: The Cost of Minimizing Mortality, Later-stage Diagnoses
Worldwide, almost 70% of cancer deaths occur in countries ranking as medium or low on the Human Development Index. Poverty, including less access to education and healthcare, exposes residents to a greater risk of developing and dying from cancer. According to the World Health Organization, in low-income and high-income countries, fewer than 30% and more than 90% of patients diagnosed with cancer have access to treatment, respectively.4
At the same time, the development of cancer in an individual impacts productivity and family income because of the high costs of treatment, which can impoverish a family.
Published studies on COVID-19 and cancer show that patients with active neoplasms have a higher risk of severe complications and mortality from SARS-CoV-2 infection than the general population, particularly those with lung cancer, on myeloid suppressive treatments, and/or with advanced age, compromise of their functional status, or comorbidities. Added to the risk of dying from severe complications is the risk resulting from the overflow of the health system in the context of a severe outbreak as well as delays in the care of cancer and other diseases due to the measures implemented to contain the COVID-19 pandemic. Indeed, the immediate demands of the COVID-19 pandemic have required health systems to focus on containment strategies to minimize mortality. Our collective regional prioritization of COVID-19 and implementation of physical distancing as an intervention strategy has impaired cancer health providers’ functioning, specifically by postponing cancer screening, in-person consultations, and control tests and limiting treatments that might result in significant risk reduction. The impact of public health measures to contain the pandemic is interwoven with the changes in habits, and healthy behaviors as well as the consequences of the economic crisis, all of which increase poverty and create challenges for patients in accessing cancer screening and treatment promptly.5
,6
Three recent studies from the United Kingdom estimated the possible increase in cancer mortality due to the pandemic.7
,8
,9
Lai et al.7
reported a 76% reduction in the number of patients referred for a possible cancer diagnosis and a 60% reduction in chemotherapy treatments compared to pre–COVID-19 levels. The study concludes that in the 12 months following the pandemic, mortality could increase by 20% to 30% in patients with cancer. Sud et al.8
used observational studies to generate daily risk rates for cancer progression and applied them to survival by age and disease stage. They estimated that a delay per patient of 3 or 6 months would cause the attributable deaths of 4,755 and 10,760 patients, respectively, among the 80,406 long-term surviving patients (taken from the total number of patients operated on annually in the United Kingdom who have the most common invasive cancers in adults). On the other hand, Maringe et al.9
calculated the possible increase in mortality 5 years after the diagnosis of lung cancer to be 4.8 to 5.3 times higher with respect to pre-pandemic mortality.
Recently, Ruiz-Patiño et al.10
estimated that with the present incidence tendency, Latin America would be expected to lose approximately 111,725 patients with cancer to SARS-CoV-2 (range: 87,116 to 143,154) by the end of the outbreak. Losses calculated vary between 1% and 17.6% of all patients with cancer in the region. Furthermore, Ferrari et al.11
showed that the mortality from COVID-19 in a cohort of Brazilian patients with cancer was 16.7% (95% CI: 11.9-22.7), with higher rates for those 60 years or older and current or former smokers, and those with comorbidities, lung cancer, and management in a noncurative setting. In addition to the negative impact on survival, diagnosis in later stages will produce a significant increase in cancer care costs compared to those pre-pandemic. This is not minor, given the significant economic impact of cancer. Indeed, the total annual economic cost of cancer in 2010 was estimated at approximately US $1.16 trillion globally, and is increasing.3
Although it impacts all countries, it primarily affects those with the lowest incomes. Fig. 1 includes the estimated age-standardized mortality rates for selected countries (low and middle income) from Africa and Asia in 2020, adapted to the pandemic.
Fig. 1 Estimated Age-Standardized Mortality Rates (ASR; Low- and Middle-Income Countries in Africa and Asia; Lung Cancer [LC], Both Sexes, All Ages), Adjusted to the Pandemic Period
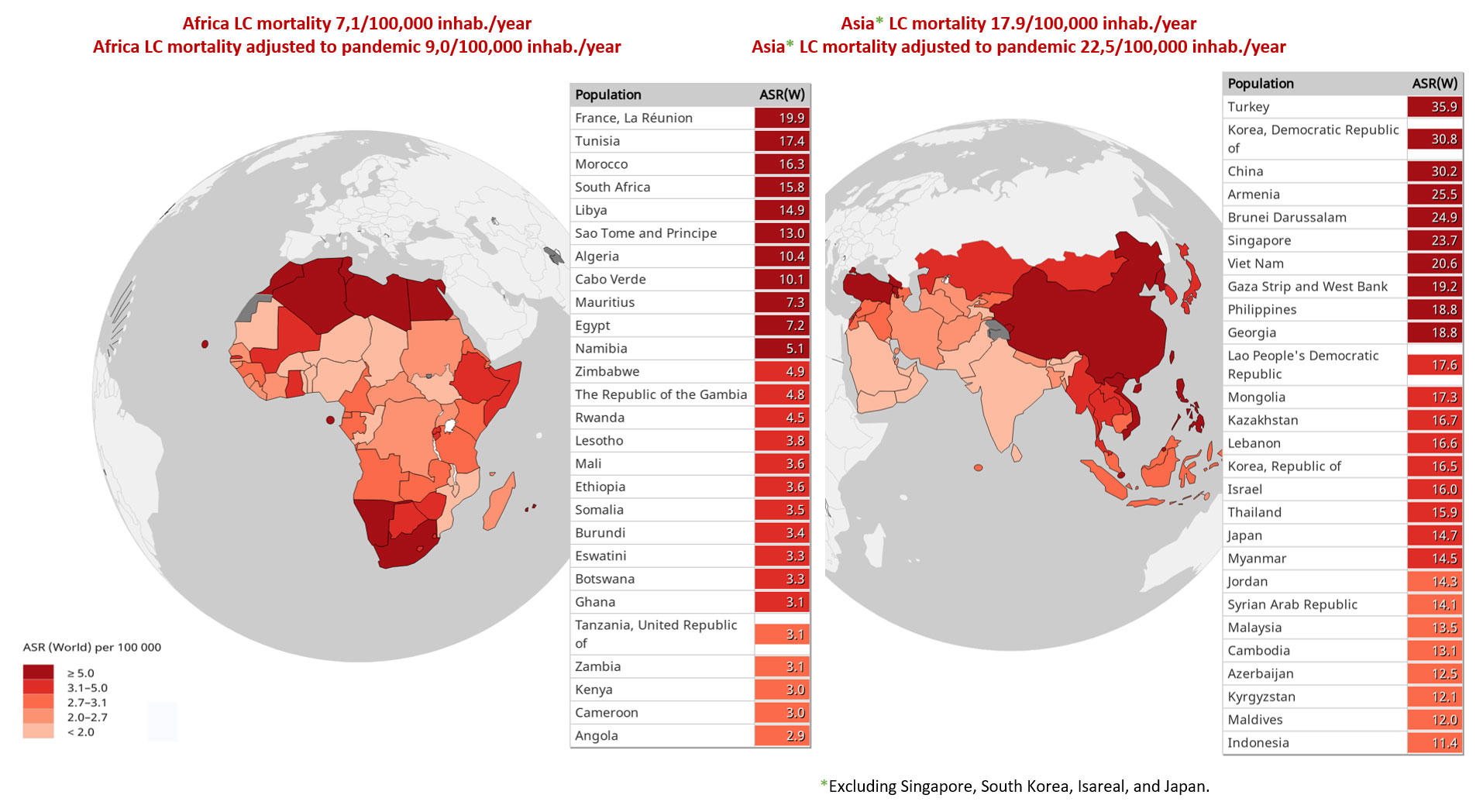
Data source: Global Cancer Observatory database.4
Data are calculated from estimates of COVID-19 mortality in patients with lung cancer, using as a basis the results of the UKCCMP project12
and the TERAVOLT study.13
Abbreviations: inhab., inhabitants.
A recent Australian study estimated the excess mortality and the economic impact resulting from diagnostic and treatment delays for four cancers (breast, lung, colorectal, and melanoma) due to the COVID-19 pandemic.14
To do this, they used a “stage change” model of the disease (available at https://cancerhealthservices.shinyapps.io/oncology_stage_shift/). Considering a 3- to 6-month delay in diagnosis and initiation of treatment, this study predicts approximately 90 to 350 excess deaths and a total of A$12 million to A$46 million (Australian dollars; US $8.6 million to US $33 million) in healthcare costs in Australia at 5 years for patients diagnosed with these four cancers in 2020.14
Fig. 2 includes lung cancer mortality per 100,000 inhabitants for nine selected Latin American countries, adjusted to the pandemic period.
Fig. 2 Lung Cancer Mortality (M) per 100,000 Inhabitants (I) for Nine Selected Latin American Countries, Adjusted to the Pandemic Period
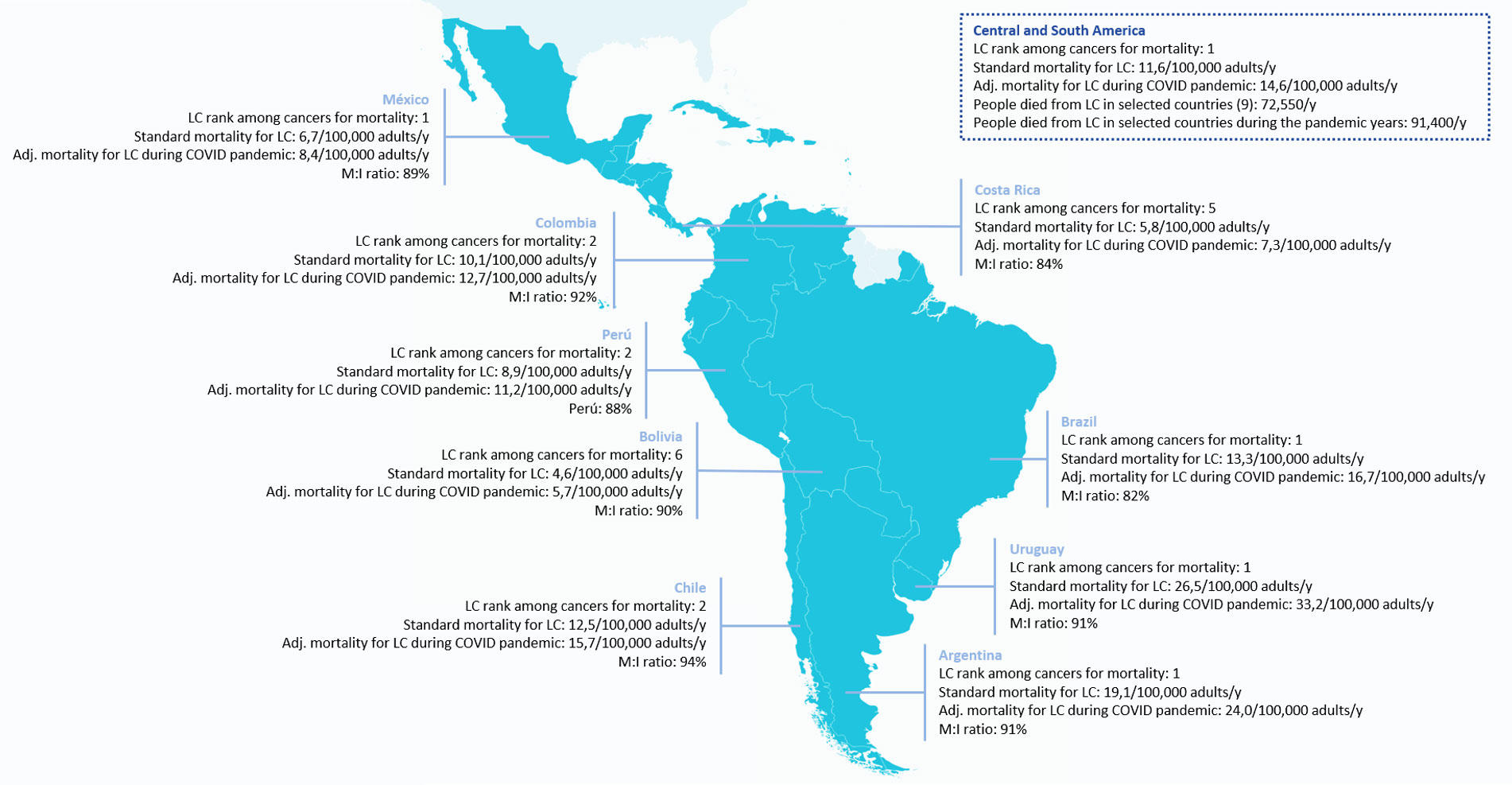
Mortality was modeled on the basis of the results of the UKCCMP project12
and the TERAVOLT study13
and adjusted from Koehring,15
which included calculations for 2012 and trends with a time horizon of 5 years.
Abbreviations: Adj., adjusted; LC, lung cancer; y, year.
Stage III NSCLC: Durvalumab Cost Considerations
Patients with stage III NSCLC are a heterogeneous group for whom treatment presents a significant challenge because of the disease’s locally advanced presentation. This is especially so in the case of an advanced primary tumor (T4) with local infiltration of vital mediastinal organs or involvement of locoregional mediastinal lymph nodes (N2 or N3 nodes), all of which poses a high risk of metastatic recurrence.16
Approximately 30% of patients affected with NSCLC are diagnosed with locally advanced disease, and despite treatment evolution, the OS is approximately 36%, 26%, and 13% for stages IIIA, IIIB, and IIIC, respectively, after concomitant or sequential chemoradiation.17
On the basis of the results of the randomized PACIFIC trial that compared durvalumab for 12 months with placebo, for patients with unresectable stage III NSCLC whose disease had not progressed on concurrent platinum-based chemoradiotherapy, durvalumab has been approved for the treatment of such patients by the U.S. Food and Drug Administration and the European Medicines Agency.18
Patients receiving durvalumab sustained a 10.7% improvement in OS (66.3%; 95% CI: 61.7%, 70.4%) compared with placebo (55.6%; 95% CI [48.9% to 61.8%]; p = 0.005). At a median follow-up of 25.2 months, the median OS was 28.7 months for the placebo arm and had not been reached for durvalumab.19
The updated results at a median follow-up of 33.3 months showed that OS had still not been reached in the durvalumab arm, compared with 29.1 months in the placebo arm (stratified HR, 0.69; 95% CI [0.55 to 0.86]).20
,21
Durvalumab is currently approved to treat locally advanced NSCLC in more than 30 countries, including several in Latin America. However, the controversy about the outcomes derived from the PACIFIC study in real practice has become evident. Recently, the international, observational PACIFIC-R study included 1,193 patients from 10 high-income countries. McDonald et al.22
noted that there were no significant differences in baseline characteristics between the concurrent and sequential chemoradiotherapy (CRT) cohorts, except for those who were 70 years of age or older (28.4% on concurrent CRT vs. 37.3% on sequential CRT) and for longest dimension of the primary tumor (15.8 mm vs. 25.2 mm, respectively). Additionally, only 68.6% of patients were tested for PD-L1 expression. A notable heterogeneity was found in the type of platinum-based chemotherapy by country and its median duration. Also, some recent data have shown that only 50% of patients with stage III lung cancer meet PACIFIC criteria for durvalumab eligibility, primarily because of disease progression during platinum-based CRT, therapy-related pneumonitis, and PD-L1 tumor proportion score less than 1% (according to the European Medicines Agency drug approval).23
,24
Stage III NSCLC: The Cost of Consolidated Therapy, Before and During the Pandemic
Regarding the economic impact of consolidation with durvalumab in stage III NSCLC, Criss et al.25
explored its cost-effectiveness and budgetary consequence in the context of the U.S. healthcare system. In a simulation of 2 million patients, durvalumab consolidation therapy was cost-effective compared with no consolidation therapy at a willingness-to-pay threshold of US $100,000 per quality-adjusted life-year, with an estimated incremental cost-effectiveness ratio of US $67,421 per quality-adjusted life-year, and would contribute an additional US $768 million to national cancer spending in Year 1. The annual budgetary consequence would then decrease to US $241 million in Year 5.25
However, for low- and middle-income countries, the willingness-to-pay threshold for health gain does not exceed US $20,000 to $30,000 per quality-adjusted life-year.26
With this consideration, only 1or 2 out of every 10 patients with stage III NSCLC could receive CRT and consolidation with durvalumab in Latin American countries. The COVID-19 pandemic will only exacerbate these inequalities by threatening the infrastructure and capacity of health systems in the region. Considering that the number needed to treat with durvalumab as consolidation after CRT in stage III NSCLC is 7 (i.e., the number of patients you need to treat to prevent one additional death),27
it is likely that the investment in treating a single patient with these conditions in Latin America covers about 4,400 doses of vaccines for SARS-CoV-2, or 2,200 people protected.
In Colombia, stage III NSCLC corresponds to 18.5% of the incident cases, but there is a treatment registry for only 54% of these patients, with no data available regarding the use of durvalumab.28
We reviewed an open national database of individual registries of medical services (RIPS), comparing the periods from March to December 2020 against March to December 2019, and found variable decreases in the number of people with services related to lung cancer, ranging from 36% to 52%, depending on their type of health insurance, with a total of 7,503 fewer people attended during this period (unpublished data; Fig. 3). Most of these patients have advanced disease (> 80%), but the rest will lose the opportunity for cure or for exposure to CRT and immunotherapy. In a severe pandemic scenario characterized by reduced resources, if patients must be triaged, essential considerations for the role of CRT in stage III NSCLC include the potential for cure, the relative benefit of radiation, life expectancy, and performance status. Case-specific consensus recommendations regarding multimodality treatment strategies and fractionation of radiotherapy should be considered.31
Fig. 3 Decrease in Number of Patients Treated with Thoracic Malignancies in Colombia (March to December 2020 vs. March to December 2019).29
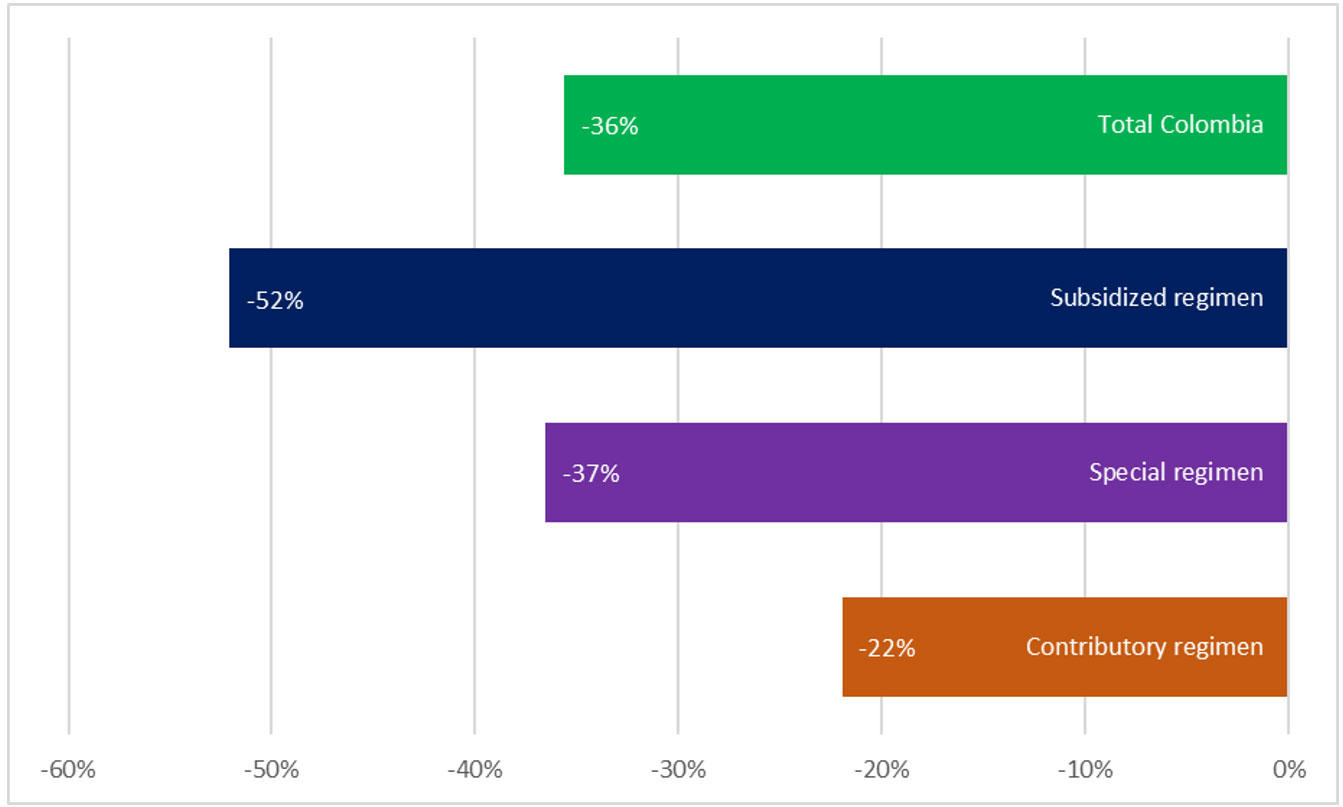
The special regimen includes public services such the armed forces, national police, national railways, and teachers’ unions. The most significant decrease in access to care and diagnosis of chest tumors is for the subsidized regimen, a social strata of people who earn between US $33 and US $440 monthly in Colombia. More than 30 million people belong to the vulnerable middle class, multidimensional poverty is 19.6%, and the COVID-19 crisis for these groups is of more significant impact. All Colombians in the poverty-vulnerability band had a high risk of falling into poverty after 1 year of the pandemic. The economic reactivation plan in Colombia has been calculated at Col$50 trillion to Col$60 trillion (Colombian pesos; 3% to 6% of GDP), whereas the United States and the European Economic Community have proposed a total economic investment for the reactivation that ranges between 22% and 25% of the GDP. The economic growth calculated by FEDESARROLLO for 2020 was 3.5% (before the start of the pandemic)30
; today, it is 2.5% in the best scenario, and 0.4% in the worst. Others have projected a regional decrease in GDP of 3.9% and an unemployment rate for Colombia near 19%.
The recent ESTRO–ASTRO consensus for lung cancer radiotherapy during the COVID-19 pandemic suggested for the later pandemic period that patients with stage III NSCLC be divided according to the fractionation provided for those treated with radiation therapy alone, with sequential CRT, or with concomitant CRT. In general, there was a strong consensus that hypofractionated radiotherapy is appropriate when treating with radiotherapy alone or sequential CRT; however, there was a consensus against hypofractionation when instituting concomitant CRT. For these cases, various fractionations were considered appropriate, with total doses between 50 and 66 Gy delivered in 15 to 30 fractions.31
These recommendations are perfectly extrapolated to patients with NSCLC in low- and middle-income countries, reducing congestion at care facilities, optimizing equipment operation, balancing the absence of suitable personnel, and reducing hospital exposure time for patients with multiple risk factors for COVID-19.
As we continue to battle the COVID-19 pandemic, delays in making a lung cancer diagnosis and the forced triage of cancer patients in regions of the world where resources remain inadequate are the difficult realities with which we must contend. The physical and financial burden provoked by the pandemic on the treatment of patients with lung cancer is troubling and incredibly challenging.
Acknowledgment: The authors acknowledge the contribution of Marcela Pérez (PhD fellow and researcher from the National University of Colombia, Bogotá, Colombia) in obtaining the information to estimate the frequency and distribution of lung cancer diagnosis in Colombia during the COVID-19 pandemic.
- 1. a. b. World Health Organization. Non communicable diseases. April 13, 2021. Accessed April 28, 2021. https://www.who.int/es/news-room/fact-sheets/detail/noncommunicable-dis…
- 2. Pan American Health Organization. Cancer. Accessed April 28, 2021. https://www.paho.org/hq/index.php?option=com_content&view=article&id=29…
- 3. a. b. World Health Organization. Cancer. Accessed April 28, 2021. https://www.who.int/news-room/fact-sheets/detail/cancer
- 4. a. b. International Agency for Research on Cancer. Global Cancer Observatory. Accessed April 28, 2021. https://gco.iarc.fr
- 5. Raymond E, Thieblemont C, Alran S, Faivre S. Impact of the COVID-19 outbreak on the management of patients with cancer. Target Oncol. 2020;15(3):249-259.
- 6. Maruthappu M, Watkins J, Noor AM, et al. Economic downturns, universal health coverage, and cancer mortality in high-income and middle-income countries, 1990-2010: a longitudinal analysis. Lancet. 2016;388(10045):684-695.
- 7. a. b. Lai A, Pasea L, Banerjee A, et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv. June 1, 2020. https://www.medrxiv.org/content/10.1101/2020.05.27.20083287v1
- 8. a. b. Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065-1074.
- 9. a. b. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023-1034.
- 10. Ruiz-Patiño A, Arrieta O, Pino LE, et al. Mortality and advanced support requirement for patients with cancer with COVID-19: a mathematical dynamic model for Latin America. JCO Glob Oncol. 2020;6:752-760.
- 11. Ferrari BL, Ferreira CG, Menezes M, et al. Determinants of COVID-19 mortality in patients with cancer from a community oncology practice in Brazil. JCO Glob Oncol. 2021;7:46-55.
- 12. a. b. Lee LY, Cazier JB, Angelis V, et al.; UK Coronavirus Monitoring Project Team. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. Published correction appears in Lancet. 2020;396(10250):534.
- 13. a. b. Garassino MC, Whisenant JG, Huang LC, et al.; TERAVOLT Investigators. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914-922.
- 14. a. b. Degeling K, Emery N, Franchini J, et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia Pac J Clin Oncol. February 10, 2021. [Epub ahead of print].
- 15. Koehring M. Lung cancer in Latin America: time to stop looking away. The Economist Intelligence Unit. September 17, 2018. Accessed April 29, 2021.
- 16. Casal-Mouriño A, Ruano-Ravina A, Lorenzo-González M, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 2021;10(1):506-518.
- 17. Huber RM, De Ruysscher D, Hoffmann H, et al. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev. 2019;28(152):190024.
- 18. Antonia SJ, Villegas A, Daniel D, et al.; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
- 19. Antonia SJ, Villegas A, Daniel D, et al.; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018379(24):2342-2350.
- 20. Gray JE, Villegas A, Daniel D, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC—update from PACIFIC. J Thorac Oncol. 2020;15(2):288-293.
- 21. Faivre-Finn C, Vicente D, Kurata T, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC—an update from the PACIFIC trial. J Thorac Oncol. January 19, 2021. [Epub ahead of print].
- 22. McDonald F, Mornex F, Garassino MC, et al. PACIFIC-R: real-world characteristics of unresectable stage III NSCLC patients treated with durvalumab after chemoradiotherapy. Presented at: European Lung Cancer Virtual Congress; March 25-27, 2021. Abstract 79M0.
- 23. Eichkorn T, Bozorgmehr F, Regnery S, et al. Consolidation immunotherapy after platinum-based chemoradiotherapy in patients with unresectable stage III non-small cell lung cancer-cross-sectional study of eligibility and administration rates. Front Oncol. 2020;10:586449.
- 24. Sakaguchi T, Ito K, Furuhashi K, et al. Patients with unresectable stage III non-small cell lung cancer eligible to receive consolidation therapy with durvalumab in clinical practice based on PACIFIC study criteria. Respir Investig. 2019;57(5):466-471.
- 25. a. b. Criss SD, Mooradian MJ, Sheehan DF, et al. Cost-effectiveness and budgetary consequence analysis of durvalumab consolidation therapy vs no consolidation therapy after chemoradiotherapy in stage III non-small cell lung cancer in the context of the US health care system. JAMA Oncol. 2019;5(3):358-365.
- 26. Shillcutt SD, Walker DG, Goodman CA, Mills AJ. Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. Pharmacoeconomics. 2009;27(11):903-917.
- 27. Vanneste BGL, Van Limbergen EJ, Reynders K, De Ruysscher D. An overview of the published and running randomized phase 3 clinical results of radiotherapy in combination with immunotherapy. Transl Lung Cancer Res. 2021;10(4):2048-2058.
- 28. Fondo Colombiano de Enfermedades de Alto Costo. Cuenta de Alto Costo: Libro de Situación del Cáncer en Colombia 2019. Ministerio de Hacienda y Crédito Público, Ministerio de Salúd y Protección Social, República de Colombia; 2020.
- 31. a. b. Guckenberger M, Belka C, Bezjak A, et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020;107(4):631-640.
- 29. Ministerio de Salud y Protección Social. Colombia. Sistema Integrado de Información de la Protección Social. Accessed July 27, 2021. https://www.sispro.gov.co/Pages/Home.aspx
- 30. Instituto Colombiano de Bienestar Familiar. Colombia. Fundación Para el Desarrollo Social Educativo Cultural Ambiental y en la Salud y Vida Para Colombia Fundesapac. Accessed July 27, 2021. https://www.icbf.gov.co/fundacion-para-el-desarrollo-social-educativo-c…












