Heated tobacco products (HTPs) are a growing category of novel tobacco products. They typically consist of an electronic heating component and a tobacco insert in the form of a cigarette-like stick or a capsule. Whereas conventional cigarettes rely on combustion of tobacco, HTPs aerosolize nicotine from tobacco at lower temperatures. HTPs also differ from nicotine vaping products (commonly referred to as e-cigarettes) in that they contain actual tobacco, as opposed to the tobacco-free nicotine solutions found in e-cigarettes. Although the engineering and design of contemporary devices have been modernized, HTP technology dates back decades, to products such as Eclipse and Accord. Unlike those “early-generation” HTPs, which never achieved widespread demand, contemporary HTPs have gained significant traction in a number of countries.1,2
The leading brand of contemporary HTPs is called IQOS. It is manufactured and sold by the largest tobacco company, Philip Morris International (Fig. 1).3 IQOS is currently available for purchase in 55 countries.4,5 IQOS headlines an expanding smoke-free product line from Philip Morris, as the company looks to alter its public persona from being a leading cigarette manufacturer to “delivering a smoke-free future.”6
While IQOS spread to numerous countries from 2016 to 2018, it remained unavailable in the United States, where each novel tobacco product requires authorization from the U.S. Food and Drug Administration (FDA) through Premarket Tobacco Product Applications (PMTAs). The PMTA pathway requires the manufacturer to “provide scientific data that demonstrates a product is appropriate for the protection of public health” prior to receiving marketing authorization.7 Philip Morris submitted a PMTA for IQOS on March 31, 2017, which underwent a 2-year evaluation process resulting in the FDA authorizing IQOS to the market.8 On October 1, 2019, the first IQOS store opened its doors in an Atlanta, Georgia, shopping mall (Fig. 2).
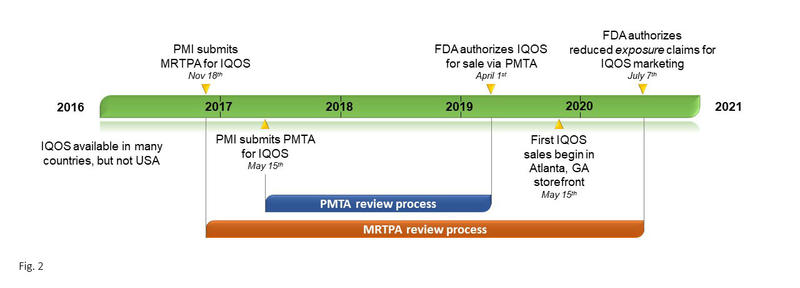
In addition to submitting a PMTA in 2017, Philip Morris had already submitted a Modified Risk Tobacco Product (MRTP) application to the FDA on November 18, 2016 (Fig. 2). MRTP authorization allows a manufacturer to make certain claims regarding modified exposure or modified risk as compared with smoking cigarettes. IQOS’s MRTP application went through its own review process, which culminated with the FDA authorizing the following modified-exposure claims on July 7, 2020: “The IQOS system heats tobacco but does not burn it”; “This significantly reduces the production of harmful and potentially harmful chemicals”; “Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals.”
The FDA simultaneously rejected two claims of reduced risk from IQOS: “Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system can reduce the risks of tobacco-related diseases” and “Switching completely to IQOS presents less risk of harm than continuing to smoke cigarettes.” The distinctions between modified exposure and modified risk are crucially important: just because a product emits fewer toxic compounds than conventional cigarettes (i.e., modified exposure) does not in and of itself confirm that a product will confer less health risk (i.e., modified risk).
Better but Not Good
Aside from MRTP semantics, there remain questions about IQOS’s applicability “for the protection of public health.” Even if using IQOS does reduce an individual user’s exposure to toxicants compared with smoking cigarettes, IQOS is certainly not a harmless product. There is always the possibility of uptake among tobacco-naive individuals (including youth), former tobacco users, or current smokers interested in using IQOS for reasons that prolong rather than stop smoking behaviors (e.g., use in settings where cigarettes are prohibited), any of which would be a negative public health development. Even among current smokers who are interested in using IQOS to help them quit, public health benefit hinges on whether quit intentions translate into actual smoking cessation over time. Understanding this requires longitudinal research in free-living populations, something that is still accumulating.
The question becomes more nuanced when considered within the complexities of the overall nicotine marketplace, most notably in relation to e-cigarettes, which appear to produce less-toxic emissions than not only conventional cigarettes but also IQOS.9–11 From here, one could argue that even if IQOS were less harmful than conventional cigarettes, it would be detrimental to public health if it attracted smokers who, in the absence of IQOS, would have tried an e-cigarette as a smoking-cessation aid. On the other hand, IQOS might attract a completely different group of smokers who aren’t interested in e-cigarettes but would switch to IQOS, which would have positive public health ramifications compared to the status quo.
Global Use Data
Who ends up using a product in the real world is difficult to fully grasp until it has been available on the market for some time. Some research has examined HTPs in the United States prior to IQOS being authorized to market, finding very little use. Moreover, the majority of HTP users also concurrently used other tobacco and nicotine products, including conventional cigarettes and e-cigarettes.12-15 How applicable these data are now, with IQOS on the market, is debatable, as they likely captured “early adopters” and are plagued by misclassification from respondents confusing HTPs with e-cigarettes.
Evidence from other countries can be informative as well. Some within the tobacco control community are cautiously optimistic about HTPs on the basis of the Japanese experience, where their growing popularity has been accompanied by accelerated declines in smoking prevalence16,17; furthermore, use of HTPs by youth18 and former tobacco users19 each appear minimal. How this translates to other countries remains unknown, particularly since potentially modified risk products (e.g., e-cigarettes, snus), other than HTPs, are uncommon in Japan. In South Korea, where the nicotine marketplace is more similar to that of the United States, the vast majority of adult HTP users were concurrently using multiple tobacco products in 2018.20 Whether such poly-tobacco use patterns are associated with smoking cessation is still unclear, but results from another 2018 South Korea survey paint a concerning picture: smokers who concurrently used other products did not differ from exclusive smokers in their intention to quit smoking.21
Only time will tell the impact HTPs have on tobacco control and public health efforts, both worldwide and within the United States. Notably, the FDA has stringent oversight authority over IQOS, something that was sorely lacking with e-cigarettes for many years. In addition to the IQOS device being sold only at product-specific “IQOS stores,” PMI must report post-market surveillance of trends, including youth uptake; marketing must explicitly target adults; and the FDA claims to be paying particularly close attention to marketing campaigns on social media platforms.22 In theory, this provides a mechanism to swiftly remove IQOS from the market if it is being used in ways that do not benefit public health. We hope future regulatory decisions will be informed by upcoming longitudinal studies examining patterns of use, smoking cessation outcomes, and continued surveillance of HTP user characteristics, as well as by epidemiologic studies and randomized trials that better assess the health consequences of HTPs.
References:
- Smokeless tobacco, e-vapour products and heated tobacco in South Korea. Euromonitor Int. July 2020. Accessed October 6, 2020. https://www.euromonitor.com/smokeless-tobacco-e-vapour-products-and-hea…
- Tobacco in Japan. Euromonitor Int. July 2020. Accessed October 6, 2020. https://www.euromonitor.com/tobacco-in-japan/report
- Jackler RK, Ramamurthi D, Axelrod AK, et al. Global marketing of IQOS: the Philip Morris campaign to popularize “heat not burn” tobacco. Stanford Research into the Impact of Tobacco Advertising. February 21, 2020. Accessed October 6, 2020. http://tobacco.stanford.edu/tobacco_main/publications/IQOS_Paper_2-21-2…
- Glo. [Country selector]. Accessed October 6, 2020. https://www.discoverglo.com
- Philip Morris International. Our tobacco heating system: IQOS. Accessed October 6, 2020. https://www.pmi.com/smoke-free-products/iqos-our-tobacco-heating-system
- Philip Morris International. Delivering a smoke-free future. Updated July 31, 2019. Accessed October 6, 2020. https://www.pmi.com/our-transformation/delivering-a-smoke-free-future
- U.S. Food & Drug Administration. Premarket tobacco product applications. Updated September 11, 2020. Accessed October 6, 2020. https://www.fda.gov/tobacco-products/market-and-distribute-tobacco-prod…
- U.S. Food & Drug Administration. FDA permits sale of IQOS tobacco heating system through Premarket Tobacco Product Application pathway. Updated April 30, 2019. Accessed October 6, 2020. https://www.fda.gov/news-events/press-announcements/fda-permits-sale-iq…
- Leigh NJ, Tran PL, O’Connor RJ, Goniewicz ML. Cytotoxic effects of heated tobacco products (HTP) on human bronchial epithelial cells. Tob. Control. 2018;27:s26-s29.
- Leigh NJ, Palumbo MN, Marino AM, O’Connor RJ, Goniewicz ML. Tobacco-specific nitrosamines (TSNA) in heated tobacco product IQOS. Tob Control. 2018;27:s37-s38.
- Farsalinos KE, Yannovits N, Sarri T, Voudris V, Poulas K, Leischow SJ. Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette. Addiction. 2018;113(11): 2099-2106.
- Miller CR, Sutanto, E, Smith, DM, et al. Awareness, trial, and use of heated tobacco products among adult cigarette smokers and e-cigarettes users: findings from the 2018 ITC Four Country Smoking & Vaping Survey. Tob Control. [Epub ahead of print].
- Dunbar MS, Seelam R, Tucker J, Rodriguez A, Shih RA, D’Amico, EJ. Correlates of awareness and use of heated tobacco products in a sample of US young adults in 2018–2019. Nicotine Tob Res. February 2020. doi:10.1093/ntr/ntaa007
- Nyman AL, Weaver SR, Popova L, et al. Awareness and use of heated tobacco products among US adults, 2016–2017. Tob Control. 2018;27(suppl 1):s55-s61.
- Marynak KL, Wang TW, King BA, Agaku IT, Reimels EA, Gaffunder CM. Awareness and ever use of “heat-not-burn” tobacco products among US adults, 2017. Am J Prev Med. 2018;55(4):551-554.
- Stoklosa M, Cahn Z, Liber A, Nargis N, Drope J. Effect of IQOS introduction on cigarette sales: evidence of decline and replacement. Tob Control. 2020;29:381-387.
- Cummings KM, Nahhas GJ, Sweanor DT. What is accounting for the rapid decline in cigarette sales in Japan? Int J Environ Res Public Health. 2020;17(10):3570.
- Kuwabara Y, Kinjo A, Fujii M, et al. Heat-not-burn tobacco, electronic cigarettes, and combustible cigarette use among Japanese adolescents: a nationwide population survey 2017. BMC Public Health. 2020;20:1-9.
- Sutanto E, Miller C, Smith DM, et al. Prevalence, use behaviors, and preferences among users of heated tobacco products: findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health. 2019;16:4630.
- Hwang JH, Ryu DH, Park S-W. Heated tobacco products: cigarette complements, not substitutes. Drug Alcohol Depend. 2019;204;107576.
- Kim SH, Cho H-J. Prevalence and correlates of current use of heated tobacco products among a nationally representative sample of Korean adults: results from a cross-sectional study. Tob Induc Dis. 2020;18:66.
- U.S. Food & Drug Administration. FDA authorizes marketing of IQOS tobacco heating system with “reduced exposure” information. July 7, 2020; Accessed October 6, 2020. https://www.fda.gov/news-events/press-announcements/fda-authorizes-mark…






