Posted: August 14, 2019 Lorlatinib, a smallmolecule inhibitor of ALK and ROS1, was granted accelerated U.S. Food and Drug Administration approval in November 2018 for patients with ALK-positive metastatic NSCLC whose […] Read more
Clinical Utility of Plasma Next-Generation Sequencing in Advanced NSCLC: Are We Ready for a ‘Blood-First’ Approach?
By Christian Rolfo, MD, PhD, MBA, Dr.h.c., and Lori Alexander, MTPW, ELS, MWC Posted: August 14, 2019 Targeted therapy has become the standard of care in patients with advanced NSCLC and […] Read more
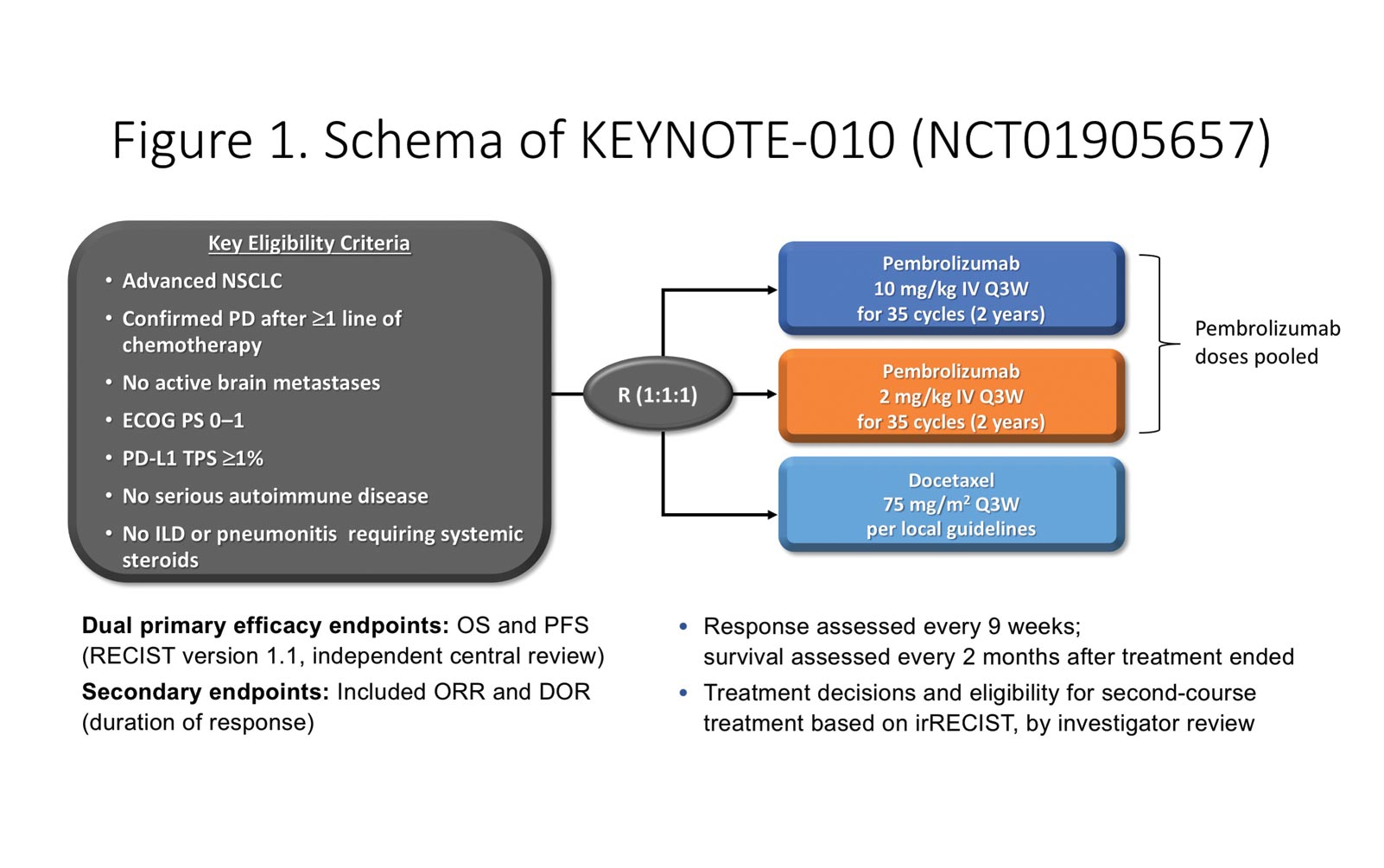
By Emily F. Collier, MD; Roy S. Herbst, MD, PhD; and Sarah B. Goldberg, MD, MPH Posted: August 14, 2019 Dr. Emily F. Collier Dr. Roy S. Herbst Dr. Sarah B. […] Read more
Posted: August 14, 2019 People diagnosed with lung cancer do not have to live close to a major cancer center to get a second opinion from one of its experts, thanks […] Read more
Posted: May 27, 2019 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial) is a large umbrella trial evaluating patients with early-stage NSCLC who have undergone complete tumor resection. As […] Read more
By Paul A. Bunn Jr., MD Posted: May 27, 2019 It is indeed an exciting time in lung cancer therapeutics, and we have reasons to be optimistic. To put these advances […] Read more
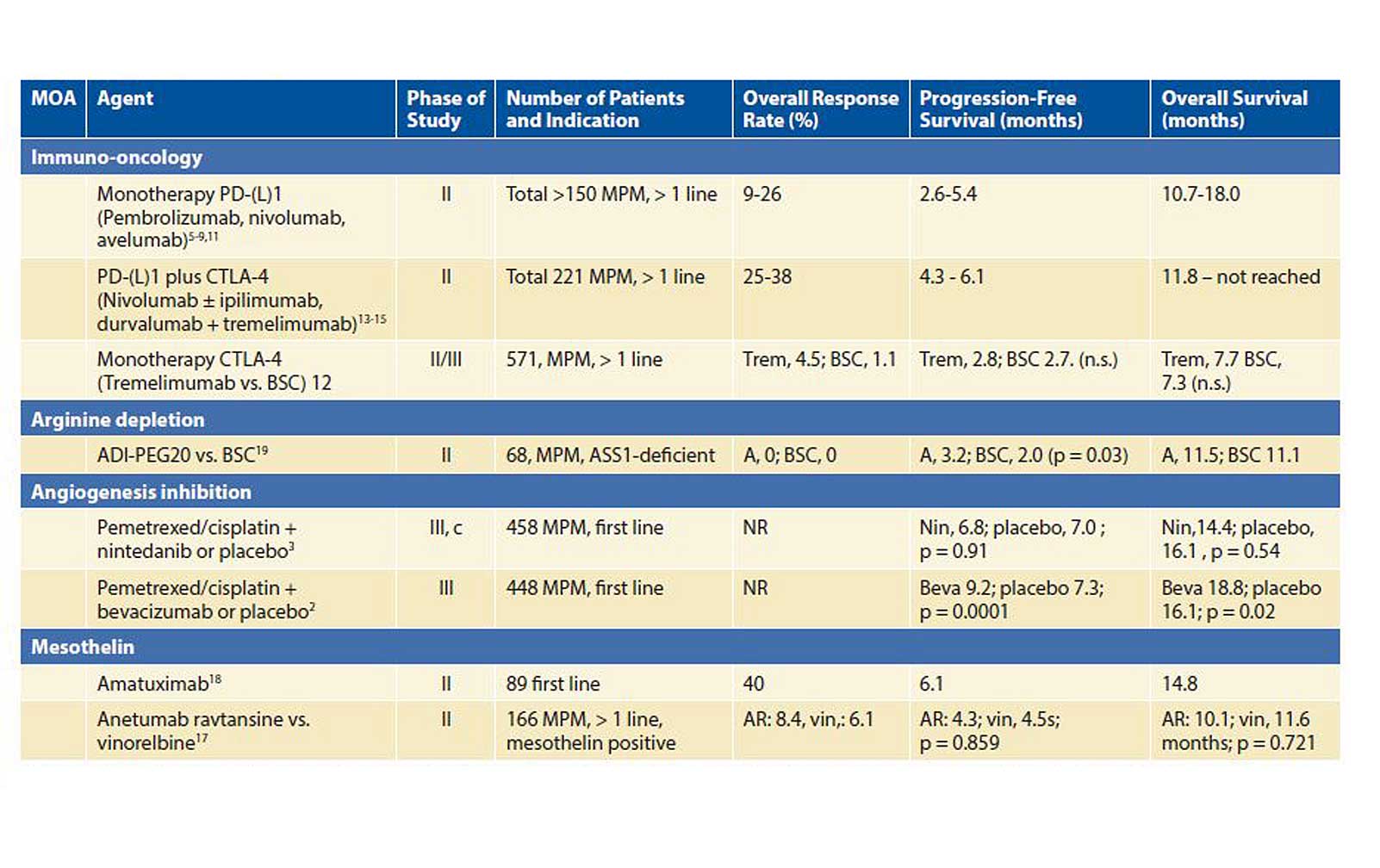
By Cornedine Jannette de Gooijer, MD; Maria Disselhorst, MD; and Paul Baas, MD, PhD Posted: May 27, 2019 Dr. Cornedine Jannette de Gooijer Until 2015, few changes occurred with respect to […] Read more
2019 IASLC Best of the World Lung Cancer Conference Results in New Virtual Collaboration
Posted: May 27, 2019 In March 2019, another successful Best of the World Lung Cancer Conference (BWLCC) was held in Lima, Peru. This was the fourth year in a row that […] Read more
Checkpoint Inhibitors and Clinical Decision Making: A Q&A with Dr. Nasser H. Hanna
Posted: May 27, 2019 Nasser H. Hanna, MD, is the Tom and Julie Wood Family Foundation Professor of Lung Cancer Clinical Research at Indiana University School of Medicine, and he specializes […] Read more
By Alice Shaw, MD, PhD Posted: May 27, 2019 On November 2, 2018, lorlatinib, a potent and central nervous system (CNS)–penetrant next-generation ALK/ROS1 inhibitor, was granted accelerated approval by the U.S. […] Read more