Surgery has long been the gold standard for diagnosis and management of early-stage operable lung cancer. For patients with inoperable disease or more extensive disease, however, alternative approaches are emerging. For example, there are ample data to support stereotactic body radiation therapy as an alternative treatment.1 Percutaneous ablation, however, despite being available for 2 decades, is still a less-accepted approach. Until recently, this may have been due to varying recurrence rates; mixed populations of primary and secondary tumors; and a combination of different thermal- and freezing-based modalities within the same study population, creating ambiguity and preventing appropriate evaluation and comparisons.2
Microwave ablation delivers continuous heating at optimal temperatures in the tumor, allowing for more complete thermal ablation in a shorter period of time independent of the surrounding tissue type. In addition, microwaves can penetrate tissues that have a high impedance to electrical current, allowing them to create larger tumor ablation fields without a heat sink effect. When a tumor is in close proximity to the vessel, the blood can cause a cooling effect and reduce the ablation volume. This has been noted in other ablation types such as radiofrequency ablation, but microwave has been shown to have faster and larger ablation volumes with less susceptibility to this effect.
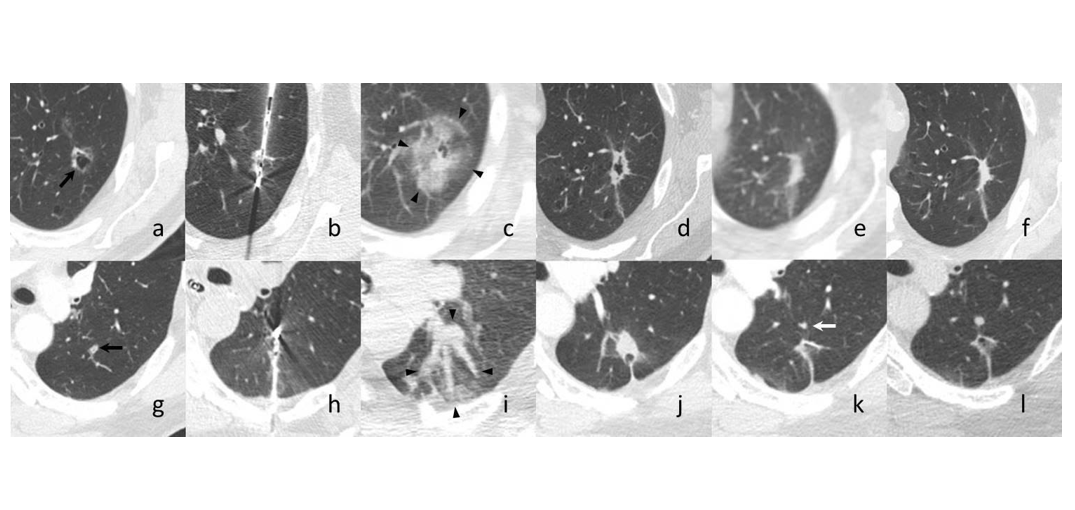
The authors recently conducted a prospective single-center trial (NCT02673021) to evaluate the role of percutaneous microwave ablation in biopsy-proven primary and secondary lung cancer. Albeit with a sample size of only seven lesions in six patients, there were no serious adverse events, and only one in-field recurrence reported in a patient with metastatic colorectal cancer at the 12-month mark (Fig.). Additionally, pulmonary function testing was captured in all patients pre- and post-procedure and was found to have a mean change of -2% in forced expiratory volume in the first second and -1% in diffusing capacity for carbon monoxide. Although small, this trial is one of very few to include a preoperative biopsy to confirm malignancy. Accompanying PET–MRI data also helped investigators understand physiology post-ablation.
These results are in concordance with the recently published MALT study by Iezzi et al.,3 in which 69 microwave ablation treatments were completed in 54 patients with primary and secondary pulmonary tumors. This study reported an OS rate of 98.0% and 71.3% at 12 and 24 months, respectively, with a local progression rate of 24.7% and average time to progression of 8.1 months (Table).
The recent emergence of electromagnetic and shape-sensing robotic platforms has propelled bronchoscopic access to pulmonary nodules by affording reach, stability, and precision of pulmonary nodule biopsy. Furthermore, seamless integration of 3D imaging to visualize “tool in lesion” mitigates concerns regarding false negative biopsies. The addition of real-time imaging allows the proceduralist to visualize the exact location of the ablation catheter relative to the ablation target, as well as obtain images during the ablation to ensure the ablation zone and ablation margins are adequate. As these platforms evolve and allow better transoral access to pulmonary nodules without the complications of hemorrhage and pneumothorax, the therapeutic potential expands.
Currently, few bronchoscopic ablation systems are being trialed for safety, feasibility, and efficacy. All involve microwave ablation, and these procedures are performed in a hybrid operating room with cone-beam CT capability and a multidisciplinary team to screen, enroll, treat, and follow the patient population. Theoretically, these systems avoid the risk of chest-wall pain and bronchopleural fistula associated with a percutaneous route, while maintaining the benefits of percutaneous ablation: preserving pulmonary function and allowing for re-treatment.
Further investigation is warranted to answer the following questions: 1) On a large scale, is bronchoscopic ablation safe and feasible? 2) How does the efficacy compare with that of other existing treatment modalities? 3) Is microwave ablation truly the best ablation mechanism compared with other forms of heat-based or non-thermal therapy? 4) Will this strategy facilitate a paradigm shift for early-stage lung cancer?
Although these questions remain unanswered for now, there is one certainty: In the near future, the lung cancer toolbox will be much more robust and diverse and much more individualized, and it will require the engagement of a large multidisciplinary team of experts to manage these patients.
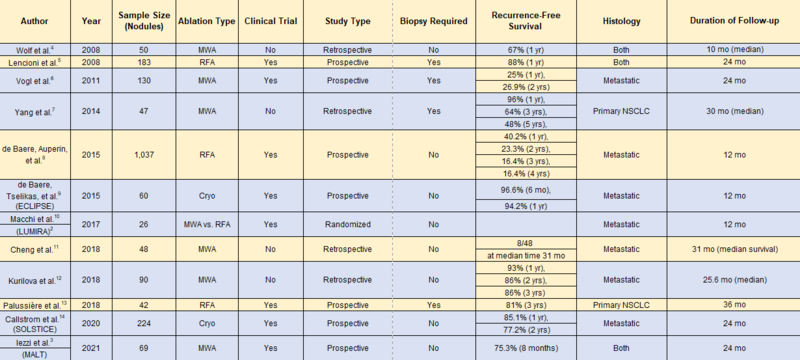
Table. Review of Literature
References:
- Van Baardwijk A, Tomé WA, van Elmpt W, et al. Is high-dose stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer (NSCLC) overkill? A systematic review. Radiother Oncol. 2012;105(2):145-149.
- Macchi, M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34(5):96.
- Iezzi, R, Cioni R, Basile D, et al. Standardizing percutaneous Microwave Ablation in the treatment of Lung Tumors: a prospective multicenter trial (MALT study). Eur Radiol. 2021;31(4):2173-2182.
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiol. 2008;247(3):871-879.
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9(7):621-628.
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiol. 2011;261(2):643-651.
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non‐small cell lung cancer: Clinical evaluation of 47 cases. J Surg Oncol. 2014;110(6):758-763.
- De Baere T, Auperin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26(5):987-991.
- De Baere T, Tselikas L, Woodrum D, et al. Evaluating cryoablation of metastatic lung tumors in patients—safety and efficacy the ECLIPSE trial—interim analysis at 1 year. J Thorac Oncol. 2015;10(10):1468-1474.
- Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34(5):96.
- Cheng G, Shi L, Qiang W, et al. The safety and efficacy of microwave ablation for the treatment of CRC pulmonary metastases. Int J Hyperthermia. 2018;34(4):486-491.
- Kurilova I, Gonzalez-Aguirre A, Beets-Tan RG, et al. Microwave ablation in the management of colorectal cancer pulmonary metastases. Cardiovasc Intervent Radiol. 2018;41(10):1530-1544.
- Palussière J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non–small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg. 2018;13(1):1-9.
- Callstrom MR, Woodrum DA, Nichols FC, et al. Multicenter study of metastatic lung tumors targeted by interventional cryoablation evaluation (SOLSTICE). J Thorac Oncol. 2020;15(7):1200-1209.