Editor’s Note: This is one of two articles exploring the implications of financial toxicity in lung cancer in today’s edition of ILCN. Read the other article here and look for more on this subject in the coming months. You can also learn more by listening to IASLC’s podcast on the subject.

Lung cancer remains the leading cause of cancer-related death worldwide, with more than two million new cases and about 1.8 million deaths in 2020.1 Fortunately, lung cancer mortality has showed an accelerated decrease both in males and females since 2013, shortly after the EGFR TKIs were introduced into standard clinical practice.2 Since then, the number of innovative anticancer drugs including targeted agents and immune checkpoint inhibitors for lung cancer treatment has been rapidly increasing.3
This treatment evolution has brought improvement in patients’ outcomes, but unfortunately, we have seen increasing financial toxicity during the same time—pointing to a need for integration of innovation and sustainability. One strategy for addressing rising treatment costs has been the implementation of diagnostic-therapeutic pathways in oncology. In 2017, Jackman et al showed the introduction of clinical pathways in metastatic NSCLC could reduce costs without compromising outcomes.4
Diagnostic-therapeutic pathways in oncology have also been endorsed and encouraged by scientific societies to promote quality and value in cancer care, to provide evidence-based treatment protocols for specific patient presentations, and to ensure cost-effectiveness of treatment strategies. Widespread availability and implementation of diagnostic-therapeutic pathways, if successful, should create a more patient-centric healthcare system based on reproducibility and uniformity in healthcare service delivery, reduction of unscheduled events and toxicities, and improved information exchange and role definition.

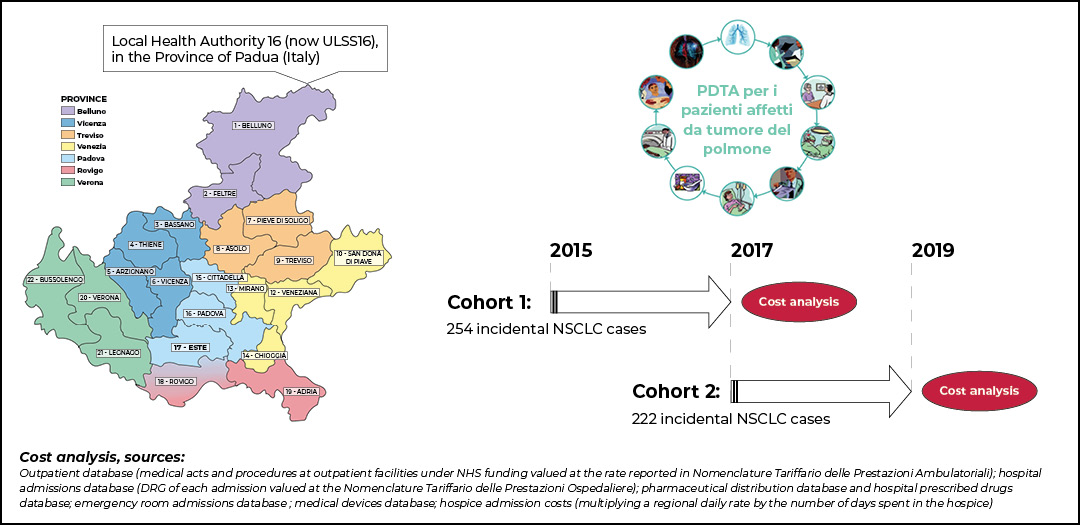
To address some of these issues, the Veneto Oncology Network was established in 2014. Lung cancer incidence and prevalence in this region of Italy account for about 3,300 and 7,600 cases yearly, respectively. The Veneto Oncology Network is built upon pivotal organizational infrastructure at the basis of diagnostic-therapeutic pathways definition, innovative drugs recommendation, and research promotion in oncology.
The network aims to support observational, real-world, and pragmatic clinical studies that have the added value of assessing safety and effectiveness of anti-cancer drugs in patients usually excluded from pivotal randomized clinical trials; these individuals are often treated in smaller centers where resources and services are limited. The network also offers an opportunity for community oncologists to play an active role in clinical research. The regional document defining the optimal diagnostic-therapeutic pathway for patients with lung cancer was approved and published at the end of 2017.
By April 2021, our group at Veneto Oncology Network—with the collaboration of Prof. Alessandra Buja from University of Padova—published an original article delineating the clinical management, patient outcomes, and costs before and after the implementation of our regional diagnostic-therapeutic pathway (PDTA).5
Anonymized data of a population cohort of incident NSCLC cases diagnosed in 2015 (n=254, pre-PDTA cohort) and 2017 (n= 228, post-PDTA cohort) were collected and analyzed. Several data sources were used to collect information about disease stage and histology, patient diagnostic procedures and locoregional and/or systemic treatments, and costs. For two years after diagnosis, data on drug prescriptions, use of medical devices, hospital admissions, visits to outpatient clinics and the emergency room, and hospice admissions were collected from the administrative database.

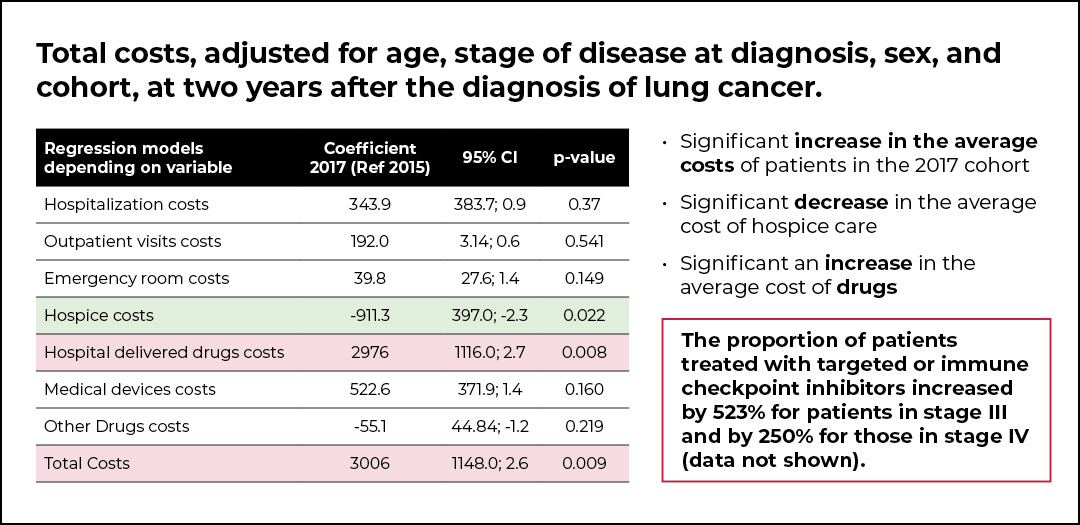
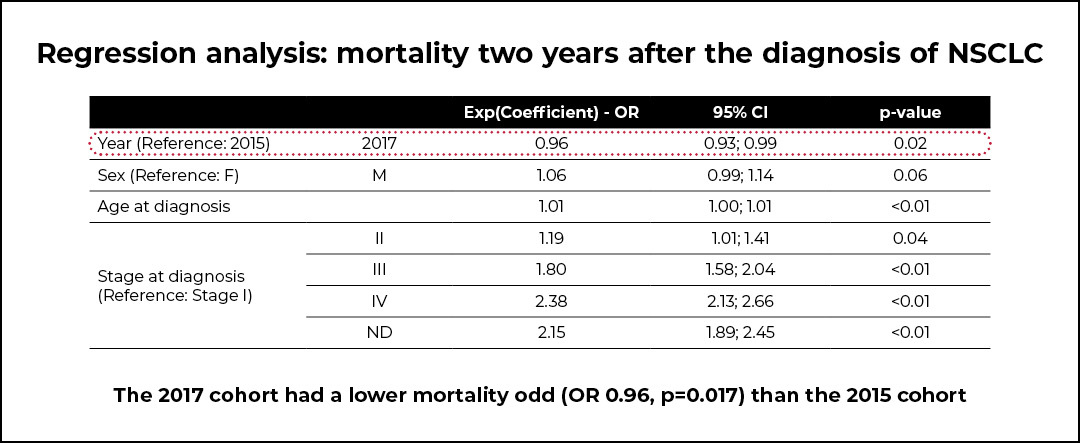
The post-PDTA cohort of patients was characterized by a lower mortality (OR 0.96, p=0.017) compared with the pre-PDTA cohort. However, a significant increase in the average cost per patient in the 2017 cohort was shown, and this was mainly due to a significant increase in the average cost of drugs. Indeed, the proportion of patients treated with targeted or immune checkpoint inhibitors increased by 523% for patients with stage III NSCLC and by 250% for those with stage IV NSCLC. Additionally, a significant decrease in the average cost of hospice care was observed.

Real-world studies have become an essential tool in evidence-based medicine since they provide the scientific community with clinical, diagnostic, therapeutic, and economic data, which are difficult to assess in randomized clinical trials; these sorts of studies have been suggested by scientific societies and regulatory agencies to be an important step to include in an innovative drug development process. Administrative health flows data refer to anonymous information tracked by regional or national governments for administrative purposes. While not originally intended for research, administrative health flows can be used by different providers as a source of information for real-world studies.
Our findings at Veneto Oncology Network provide stakeholders with a useful model of clinical and economical information on a diagnostic-therapeutic pathway of lung cancer patients.
With the primary aim of expanding such a model to a wider patient population and to update data on survival and costs, including the most recent anticancer drugs introduced in Italian clinical practice, the authors are currently implementing a multicenter observational cohort study including a retrospective collection of data on patients diagnosed with NSCLC in the whole Veneto region. This study aims to assess and update the impact of introducing a specific diagnostic-therapeutic pathway for this disease on the health outcomes, actual costs, and quality of care associated with the treatment and follow-up of patients with NSCLC.
References
- 1. Sung H, Ferlay J, Siegel R, et al. Cancer. 2021;71(3):209-249. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. doi:10.3322/caac.21660
- 2. Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med. 2020;383(7):640-649. doi:10.1056/NEJMoa1916623
- 3. Mullard A. 2020 FDA drug approvals. Nat Rev Drug Discov. 2021;20(2):85-90. doi:10.1038/d41573-021-00002-0
- 4. Jackman DM, Zhang Y, Dalby C, et al. Cost and Survival Analysis Before and After Implementation of Dana-Farber Clinical Pathways for Patients With Stage IV Non-Small-Cell Lung Cancer. J Oncol Pract. 2017;13(4):e346-e352. doi:10.1200/JOP.2017.021741
- 5. Buja A, Pasello G, De Luca G, et al. Non-Small-Cell Lung Cancer: Real-World Cost Consequence Analysis [published correction appears in JCO Oncol Pract. 2021 Nov;17(11):694]. JCO Oncol Pract. 2021;17(8):e1085-e1093. doi:10.1200/OP.20.00863