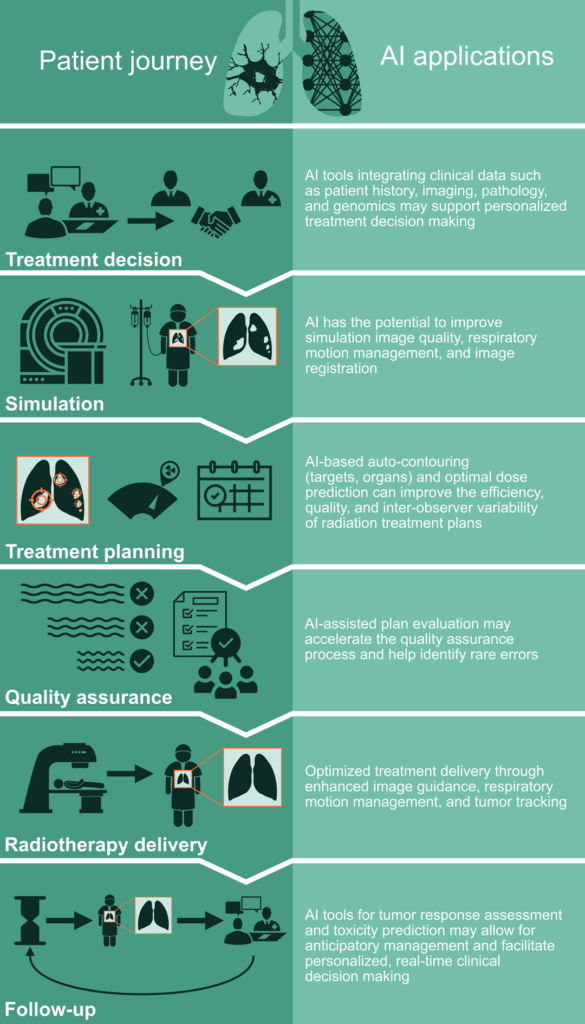
Author’s Note: This is the second part of a 2-part examination of the potential applications of artificial intelligence in thoracic radiation oncology (Figure 1). Part 1 explored patient evaluation and treatment planning. Additionally, our previous article, Demystifying AI: Current Insights on Artificial Intelligence in Thoracic Oncology, laid the groundwork for the discussions that follow.
With rapidly increasing development and integration of artificial intelligence (AI) in the field of radiation oncology and the treatment of lung cancer, clinicians need to be well-versed in the AI landscape and its tools. The integration of AI in radiation oncology has the potential to reshape provider roles, shifting focus from manual tasks to ensuring the quality of AI-generated parameters and enhancing the quality of patient care.
Part 2 of our look at AI’s impact on thoracic radiation oncology focuses on optimizing treatment delivery, post-treatment management, and navigating extensive datasets. We also look ahead at what may be coming as AI continues to evolve.
Treatment Delivery
Delivery of RT is a crucial step that requires precise and accurate alignment of the patient, tumor, and nearby organs. Cone beam CT (CBCT) imaging on the treatment machine is most often employed for patient positioning, and AI-based imaging augmentation techniques have been shown to be capable of improving the image quality of CBCT for better patient alignment.1 Additionally, patient-specific AI models capable of predicting respiratory motion and tumor position can allow for enhanced tumor tracking and more precise dose delivery.2,3
Post-Treatment Care
Tumor Response
Assessment of tumor response to RT in the lung can be challenging given the limitations of commonly used assessment criteria based on radiographic size and Response Evaluation Criteria in Solid Tumors (RECIST),4 especially in the setting of RT-induced lung changes.5 AI-driven radiomics via the quantitative assessment of post-RT CT images in lung cancer have been shown to predict tumor response to treatment, cancer-specific outcomes such as locoregional or distant recurrence, and overall survival.6,7 Such AI-guided evaluation of post-treatment images may facilitate more precise distinction between recurrence versus radiation-induced lung changes as well as early detection of recurrences that allow for early salvage therapy planning.

Toxicity
Management of acute and late radiation-induced toxicities is an important component of post-treatment care in lung cancer patients who are at risk for toxicities such as esophagitis, pneumonitis, and cardiovascular events.8 AI models predicting risk of radiation toxicities may allow for anticipatory management and secondary prevention during and after treatment. To this point, AI-based models for predicting radiation-induced pneumonitis and esophagitis9,10 have been developed. Moreover, machine learning techniques based on routinely collected pretreatment electronic medical record (EMR) data have been shown to predict emergency visits and hospitalization during RT.11 Application of such AI tools may yield real-time, actionable prediction of adverse events during and post-RT in lung cancer patients.
Challenges: Regulation and Quality Assurance of AI in Radiation Oncology
Most AI applications in radiation oncology are regulated under the Software as a Medical Device (SaMD) category per the US Food and Drug Administration (FDA) or similar standards by other international regulatory bodies.12,13 Despite a significant increase in AI-related medical publications as well as FDA-approved AI tools, the clinical implementation of AI tools in radiation oncology, and in medicine overall, has been somewhat limited, although there has been a substantial increase in the commercial availability of auto-segmentation algorithms (predominantly for organ segmentation) in radiation oncology clinics. Rigorous clinical validation and post-marketing monitoring is needed to ensure patient safety and trust, and robust quality assurance frameworks to safely implement these burgeoning AI tools. As AI-driven automation becomes increasingly common in radiation oncology, careful attention to human factors including automation complacency and skill loss will be needed to prevent missteps that have been observed in other assistive/automation AI deployments such as with “self-driving” cars.

Additionally, the lack of external validation for many models and the opaque, “black box” nature inherent in most AI algorithms, which obscures their decision-making processes, may lead to unique quality and safety concerns in the radiation oncology workflow. This may include unrecognized failure modes such as mistargeting when deploying automated segmentation algorithms beyond the intended use onto cases that are outside the algorithm’s training data. Alternatively, automated AI-based RT plans could lock onto average (or historic) outcomes due to population-based training data. For example, training an AI-based automated treatment planning algorithm for lung cancer RT with data from the early 2000s would reinforce high cardiac dose exposures given practice patterns in that era. Another possibility includes outlier clinical outputs, which could happen if an auto-segmentation algorithm is based on segmentations from a single clinician.
Furthermore, any potential systemic biases inherent in many training sets can exacerbate existing inequities in lung cancer outcomes. The maturation of these technologies will require standardization of how clinicians are informed about the training, validation, and pitfalls of a given clinical algorithm,14 and national guidelines on safe clinical implementation.15

Future Directions
The role of AI in the field of radiation oncology and lung cancer is rapidly evolving, with key developments and challenges lying ahead. Multimodal models consolidating patient data streams, including imaging and genomic markers, are needed to better understand radiation sensitivity and immunotherapy-susceptibility of lung cancer. Development of foundation AI models trained on vast datasets and generalist medical AI models that extend these foundations across various clinical tasks will offer adaptability and better generalizability of AI algorithms for lung cancer.
Generative AI, capable of creating new content through systematic training on existing datasets, holds potential for generating medical images and data for training of AI model development specific to lung cancer with incorporation of more recent advances in therapeutics such as immunotherapy. Additionally, while prior AI research in radiation oncology has mostly focused on the readily available patient outcome data, such as overall survival, there is an increasing need to focus on patient-centric outcomes directly related to RT such as radiation-induced toxicities, including high-grade cardiopulmonary toxicity, which are particularly important to patients’ well-being and quality of life.
References
- 1. Kida S, Nakamoto T, Nakano M, et al. Cone Beam Computed Tomography Image Quality Improvement Using a Deep Convolutional Neural Network. Cureus. 2018;10(4):e2548. doi:10.7759/cureus.2548
- 2. Isaksson M, Jalden J, Murphy MJ. On using an adaptive neural network to predict lung tumor motion during respiration for radiotherapy applications. Med Phys. 2005;32(12):3801-3809. doi:10.1118/1.2134958
- 3. Murphy MJ, Pokhrel D. Optimization of an adaptive neural network to predict breathing. Med Phys. 2009;36(1):40-47. doi:10.1118/1.3026608
- 4. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer. 2009;45(2):228-247. doi:10.1016/j.ejca.2008.10.026
- 5. Gulstene S, Lang P, Melody Qu X, et al. What is the predictive value of RECIST criteria following stereotactic lung radiation? Radiother Oncol. 2023;190:109976. doi:10.1016/j.radonc.2023.109976
- 6. Mattonen SA, Palma DA, Johnson C, et al. Detection of Local Cancer Recurrence After Stereotactic Ablative Radiation Therapy for Lung Cancer: Physician Performance Versus Radiomic Assessment. Int J Radiat Oncol Biol Phys. 2016;94(5):1121-1128. doi:10.1016/j.ijrobp.2015.12.369
- 7. Xu Y, Hosny A, Zeleznik R, et al. Deep Learning Predicts Lung Cancer Treatment Response from Serial Medical Imaging. Clin Cancer Res. 2019;25(11):3266-3275. doi:10.1158/1078-0432.CCR-18-2495
- 8. Bucknell NW, Belderbos J, Palma DA, et al. Avoiding Toxicity With Lung Radiation Therapy: An IASLC Perspective. Journal of Thoracic Oncology 2022;17(8):961-973. doi:10.1016/j.jtho.2022.05.003
- 9. Moran A, Daly ME, Yip SSF, Yamamoto T. Radiomics-based Assessment of Radiation-induced Lung Injury After Stereotactic Body Radiotherapy. Clin Lung Cancer. 2017;18(6):e425-e431. doi:10.1016/j.cllc.2017.05.014
- 10. Luna JM, Chao HH, Diffenderfer ES, et al. Predicting radiation pneumonitis in locally advanced stage II-III non-small cell lung cancer using machine learning. Radiother Oncol. 2019;133:106-112. doi:10.1016/j.radonc.2019.01.003
- 11. Hong JC, Niedzwiecki D, Palta M, Tenenbaum JD. Predicting Emergency Visits and Hospital Admissions During Radiation and Chemoradiation: An Internally Validated Pretreatment Machine Learning Algorithm. JCO Clin Cancer Inform. 2018;2:1-11. doi:10.1200/CCI.18.00037
- 12. Center for Devices & Radiological Health. Software as a Medical Device (SaMD). U.S. Food and Drug Administration Https://Www.Fda.Gov/Medical-Devices/Digital-Health-Center-Excellence/Software-Medical-Device-Samd (2020).
- 13. Center for Devices & Radiological Health. Artificial Intelligence and Machine Learning in Software as a Medical Device. U.S. Food and Drug Administration Https://www.Fda.Gov/Medical-Devices/Software-Medical-Device-Samd/Artificial-Intelligence-and-Machine-Learning-Software-Medical-Device (2023).
- 14. Bitterman DS, Kamal A, Mak RH. An Oncology Artificial Intelligence Fact Sheet for Cancer Clinicians. JAMA Oncol. 2023;9(5):612-614. doi:10.1001/jamaoncol.2023.0012
- 15. Rong Y, Chen Q, Fu Y, et al. NRG Oncology assessment on AI deep-learning based auto-segmentation for radiotherapy: current development, clinical consideration, and future direction. International Journal of Radiation Oncology*Biology*Physics. Published online November 2023:S0360301623080392. doi:10.1016/j.ijrobp.2023.10.033





