
While radiotherapy-associated cardiac toxicity has been a well-recognized entity for several decades, our understanding of the relationship between cardiac radiotherapy (RT) dose and specific cardiac events has accelerated in recent years. For patients with lung cancer who are exposed to high dose thoracic RT, we now have a more comprehensive understanding of the contracted timeframe in which cardiac events occur in these patients, who are often at high cardiovascular risk.1,2,3
With seminal publications in 20171,2, we learned—in contrast to historical data from breast cancer and lymphoma—that cardiac events in patients with non-small cell lung cancer (NSCLC) are occurring sooner after RT that previously appreciated, with a median onset of less than 2 years and a 2-year cumulative incidence of 11% to 23%. Whereas initial studies were limited in sample size (<130 patients each), to comprehensively characterize the scope of specific cardiac events, our group subsequently published on one of the largest cohorts of locally-advanced NSCLC patients (n=748) with detailed, standardized cardiac event characterization,3 using all cardiac clinical trial common terminology criteria for adverse event (CTCAE) types and American Heart Association/American College of Cardiology defined major adverse cardiac events (MACE; defined as cardiac death, non-fatal myocardial infarction, unstable angina, heart failure, and coronary revascularization).4 We similarly observed the common occurrence and short interval to first event, including a median time to first MACE of 19 months (10% 2-year cumulative incidence) and median time to first grade ≥3 CTCAE of only 10 months (23% 2-year cumulative incidence).

Arrhythmias Common After Lung Cancer RT
Notably, the most common CTCAE type occurring after thoracic RT is arrhythmia (128/748 patients experienced at least one grade ≥3 arrhythmia); the 2-year cumulative incidence was 12%.3 Most arrhythmias were supraventricular tachyarrhythmias (61% atrial fibrillation, 12% supraventricular tachycardia [SVT], 11% atrial flutter), and proved more common in patients with a history of pre-existing coronary heart disease (CHD).3,5
When grouped into the functional categories of atrial fibrillation/flutter, non-atrial fibrillation/flutter SVT (other SVT), ventricular tachyarrhythmias (VT), and bradyarrhythmias, the 2-year cumulative incidence of grade ≥3 events was 9.0% for atrial fibrillation/flutter, 1.8% for other SVT, 1.0% for VT, and 1.7% for bradyarrhythmias, respectively.5
By contrast, Kim et al. reported a lower rate of atrial fibrillation (AFib), with only 5% of NSCLC patients (17/321), and 4% of small cell lung cancer (SCLC) patients (9/239) reported to develop AFib following thoracic RT.6 However, each of these studies was subject to the limitations of retrospective review and potential under-capture of events.7

Is Cardiac Substructure RT Dose Associated with Functionally Distinct Arrhythmias?
Recent data have demonstrated a robust association between cardiac substructures and specific cardiac events and/or mortality in patients with NSCLC, including the left anterior descending (LAD) coronary artery with MACE and mortality,8,9 as well as other structures with mortality—including regions of the base of heart,10,11 which encompass the proximal left coronary and SA node. Further data are emerging that reveal the association between cardiac substructure RT dose and specific arrhythmia types.5,6
In a recent study by Kim et al., with automated segmentation of chambers and coronaries and manual segmentation of the sinoatrial node (SA node) and atrioventricular node, dosimetric analysis revealed that the maximum dose (Dmax) to the SA node was most predictive for AFib (but not non-AFib cardiac events) and was also associated with worse survival.6 Our group similarly observed that, adjusting for pre-existing arrhythmia and CHD, that left atrium (LA) dose was significantly associated with an increased risk of atrial fibrillation/flutter, while right atrium (RA) dose was associated with an increased risk of other SVT, and left main coronary dose was associated with an increased risk of VT and bradyarrhythmias.

The mapping of pathophysiologically distinct arrhythmias (radiation dose exposure to different cardiac substructures [i.e., SVT to atrial regions vs. ventricular arrhythmias to left ventricular blood supply]) provides a biological framework to understand and potentially mitigate cardiac toxicity from RT. However, only LA dose remained significantly associated with an increased risk of mortality, while the other dose variables were not.5 The inconsistent association between RT dose to the conduction system and survival may, in part, be explained by varying rates of co-existing acute medical conditions commonly associated with arrhythmias (i.e., pneumonia, sepsis). Together, these data are the first to show functionally distinct arrhythmia classes being associated with RT dose to discrete cardiac substructures.
Let’s Take a Beat—Are SA Node and Atrial Dose the Optimal Substructures to Predict AFib?
While aforementioned data are the first to describe the important associations between cardiac substructure RT dose and specific arrhythmia events after RT, it is critical to pause and assess what these associations may truly reflect based on our understanding of cardiac anatomy and pathophysiology.
Specifically, the SA node is the physiologic pacemaker, located at the junction of the crista terminalis and superior vena cava (SVC) in the right atrium (RA). The rapid, uncoordinated atrial activity of AFib is caused by focal spontaneous firing. The majority (80% to 94%) of ectopic beats that initiate AFib originate from the superior pulmonary veins (PV) in the left atrium (LA),12,13 more specifically within LA cardiac muscle (myocardial sleeves) extending into the proximal PV (see figure).14 By contrast, only 3% to 15% of ectopic foci originate in proximity to the SA node (e.g., SVC or crista terminalis).12,13
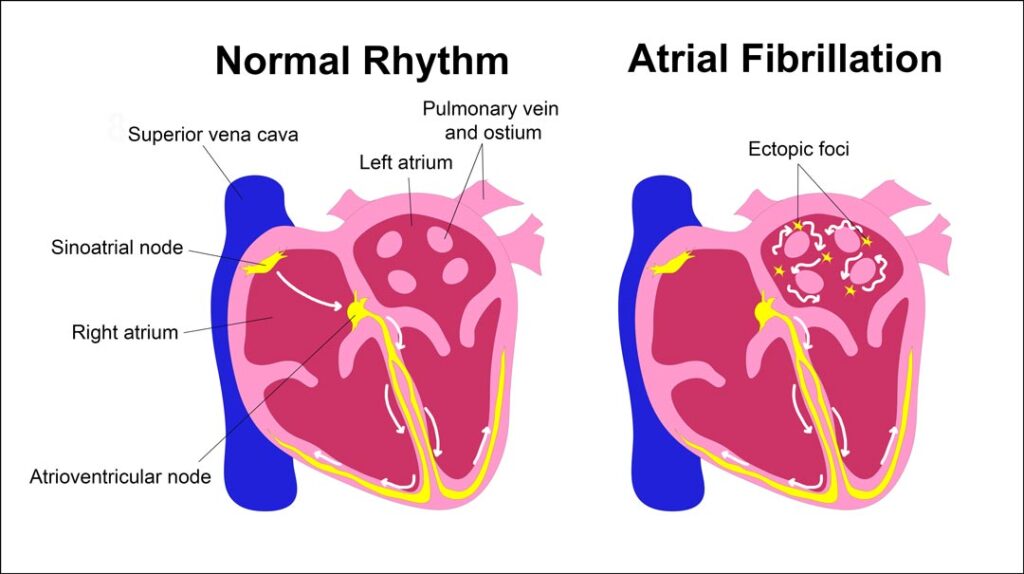
This is an important distinction—since the observation of an association between SA node RT dose and AFib may in fact be caused by the SA node being a stronger surrogate of PV dose than large atrial structures comprised predominantly of blood; in addition, the PV were not specifically segmented to measure dose exposure. Indeed, Kim et al. observed that RA and LA Dmax were also associated with an increased risk of AFib and reduced survival. The 3-year AFib rates (NSCLC cohort) stratified by cutpoint revealed similar absolute differences: SA node Dmax ≥20Gy vs. <20Gy (10% vs. 1%, p<.001), RA Dmax ≥19.1Gy vs. <19.1Gy (8% vs. 1%, p=.01), and LA Dmax ≥55.8Gy vs. <55.8Gy (9% vs 3%, p=.04).
Thus, the observation of an association between LA, RA, or SA node dose and AFib may in fact be due to these structures being a stronger surrogate of PV dose than large atrial structures. Notably, neither our study5 nor Kim et al.6 segmented PV for dosimetric analysis. Further, at the time of these studies, there were no references or cardiac contouring guidelines available for precisely delineating the PV for RT planning. Fortunately, since these reports, Walls et al.,15 have since developed a PV atlas for RT planning, which will significantly aid in future dosimetric studies for AFib.
How Can We Reduce Cardiac Risk in Our Patients?
Emerging data demonstrate that functionally distinct arrhythmia classes are associated with RT dose to discrete cardiac substructures. Together, these observations5,6 build upon our growing knowledge of cardiac substructure dose resulting in localized cardiac dysfunction and guide potential mitigation approaches. Further data suggest that precise delineation of radiation dose to the most common sites of AFib ectopic foci (e.g., proximal PVs15) may better inform optimal dosimetric predictor(s) of AFib. Importantly, continued consideration of cardiac physiology/pathophysiological mechanisms of cardiac disease for specific cardiac endpoints should be incorporated into fine-mapping cardiac substructure dose limits.
Clearly, further mechanistic work will be needed to better understand the pathway from radiation exposure to a given substructure to a specific cardiac event. The biology is likely complex, as evidenced by recent work attempting to understand the biological underpinnings of RT effect on terminating arrhythmias in patients with ventricular tachycardia.16,17 As we develop a deeper understanding of the dosimetric triggers and mechanisms of injury that lead to arrhythmias, the ability of modern RT techniques to intentionally spare critical sub-structures will likely contribute toward mitigating the risk for cardiac toxicity in future lung cancer patients.
Lastly, the lung cancer population is at exceptionally high baseline cardiovascular risk; more than 40% have known cardiovascular disease (CVD)18 and nearly 15% have pre-existing arrhythmias.3 However, even among those without known CVD—nearly half are at high Framingham risk.3 This is further coupled with the observation that less than half of these patients are being treated with cardiovascular guidelines-based medical therapy.18,19
Therefore, given this high baseline cardiovascular risk, together with the excess cardiovascular risk of multimodality treatment (including RT, chemotherapy, immunotherapy, and/or molecular targeted agents), oncologists should have a low threshold for referral to cardiology or cardio-oncology for treatment of baseline cardiac risk factors20 as well as incorporation of post-treatment surveillance.21,22,23 Further, while cardio-oncology expert panels and consensus guidelines continue to develop based on evolving data—including incorporating screening transthoracic echocardiograms and cardiac magnetic resonance imaging,21,22,23 there currently are no recommendations to screen for occult arrhythmias, nor to screen sooner for RT-associated CAD despite the shorter timeframe to developing MACE. These observations highlight the continued efforts needed to further refine screening, risk factor modification, and surveillance recommendations in these high-risk patients.
REFERENCES
- 1. Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35(13):1395–402.
- 2. Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol 2017;35(13):1387–94.
- 3. Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. J Am Coll Cardiol 2019;73(23):2976–87.
- 4. Hicks KA, Mahaffey KW, Mehran R, et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. J Am Coll Cardiol 2018;71(9):1021–34.
- 5. Atkins KM, Nikolova A, Guthier CV, et al. Association of Cardiac Sub-Structure Radiation Dose with Bradyarrhythmias and Tachyarrhythmias after Lung Cancer Radiotherapy. International Journal of Radiation Oncology*Biology*Physics 2022;114(3, Supplement):S58–9.
- 6. Kim KH, Oh J, Yang G, et al. Association of Sinoatrial Node Radiation Dose With Atrial Fibrillation and Mortality in Patients With Lung Cancer. JAMA Oncol [Internet] 2022 [cited 2022 Sep 28];Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2796765
- 7. Walls GM, Hanna GG. Sinoatrial Node Radiation Dose and Atrial Fibrillation in Patients With Lung Cancer. JAMA Oncology 2023;9(4):573–4.
- 8. Atkins KM, Chaunzwa TL, Lamba N, et al. Association of Left Anterior Descending Coronary Artery Radiation Dose With Major Adverse Cardiac Events and Mortality in Patients With Non–Small Cell Lung Cancer. JAMA Oncol 2021;7(2):206–19.
- 9. McKenzie E, Zhang S, Zakariaee R, Guthier C, Hakimian B, Mirhadi A, Kamrava M, Padda S, Lewis JH, Nikolova A, Mak RH, Atkins KM. Left Anterior Descending Coronary Artery Radiation Dose Association with All-Cause Mortality in NRG Oncology Trial RTOG 0617. ASTRO Annual Meeting 2022;
- 10. McWilliam A, Kennedy J, Hodgson C, Vasquez Osorio E, Faivre-Finn C, van Herk M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer 2017;85:106–13.
- 11. McWilliam A, Abravan A, Banfill K, Faivre-Finn C, van Herk M. Demystifying the Results of RTOG 0617: Identification of Dose Sensitive Cardiac Subregions Associated With Overall Survival. J Thorac Oncol 2023;18(5):599–607.
- 12. Lin W-S, Tai C-T, Hsieh M-H, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003;107(25):3176–83.
- 13. Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339(10):659–66.
- 14. Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100(18):1879–86.
- 15.. Walls GM, McCann C, Ball P, et al. IA PULMONARY VEIN ATLAS FOR RADIOTHERAPY PLANNING. Radiother Oncol 2023;109680.
- 16. Zhang DM, Navara R, Yin T, et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat Commun 2021;12(1):5558.
- 17. Zhang DM, Szymanski J, Bergom C, et al. Leveraging Radiobiology for Arrhythmia Management: A New Treatment Paradigm? Clin Oncol 2021;33(11):723–34.
- 18. Al-Kindi SG, Oliveira GH. Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clin Proc 2016;91(1):81–3.
- 19. Atkins KM, Bitterman DS, Chaunzwa TL, Williams CL, Rahman R, Kozono DE, Baldini EH, Aerts HJWL, Tamarappoo BK, Hoffmann U, Nohria A, Mak RH. Statin Use, Heart Radiation Dose, and Survival in Locally Advanced Lung Cancer. Practical Radiation Oncology In press 2020;
- 20. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;140(11):e596–646.
- 21. Mitchell JD, Cehic DA, Morgia M, et al. Cardiovascular Manifestations From Therapeutic Radiation: A Multidisciplinary Expert Consensus Statement From the International Cardio-Oncology Society. JACC CardioOncol 2021;3(3):360–80.
- 22. Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31(2):171–90.
- 23. Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J 2022;43(41):4229–361.




