By Mary Jo Fidler, MD
Posted: August 21, 2020
At the American Society of Clinical Oncology (ASCO) 2020 Virtual Scientific Program Plenary Session, an early look at data from the ADAURA trial revealed very promising results.1 This phase III randomized trial in patients with resected EGFR-mutated NSCLC compared post-operative osimertinib to placebo for stage IB-IIIA disease (AJCC Cancer Staging Manual, 7th Edition). Patients were not required to receive adjuvant chemotherapy, and post-operative radiation therapy was not permitted on the trial. A negative CT or MRI scan of the brain was required for entry, but routine brain imaging was not required in follow-up. The study was designed to detect superiority for a disease-free survival (DFS) with a projected hazard ratio (HR) of 0.70 in patients with stage II and IIIA disease as the primary endpoint. A 3-year duration of therapy was selected based on previously published adjuvant data with first-generation EGFR tyrosine kinase inhibitors showing early relapse after 2 years of therapy.2-5
This spring, the Independent Data Safety Monitoring Committee recommended un-blinding the study due to an early efficacy signal. At the time of un-blinding, all patients had completed at least 1 year of therapy. Importantly, patients were not un-blinded at an individual level, and they remain on their assigned therapy as of June 2020. The efficacy analysis presented at the ASCO20 Virtual Scientific Program Plenary Session was approximately 2 years ahead of schedule; information about quality-of-life measures, site of disease recurrence, and the exact numbers of patients who received post-operative chemotherapy remain unavailable.
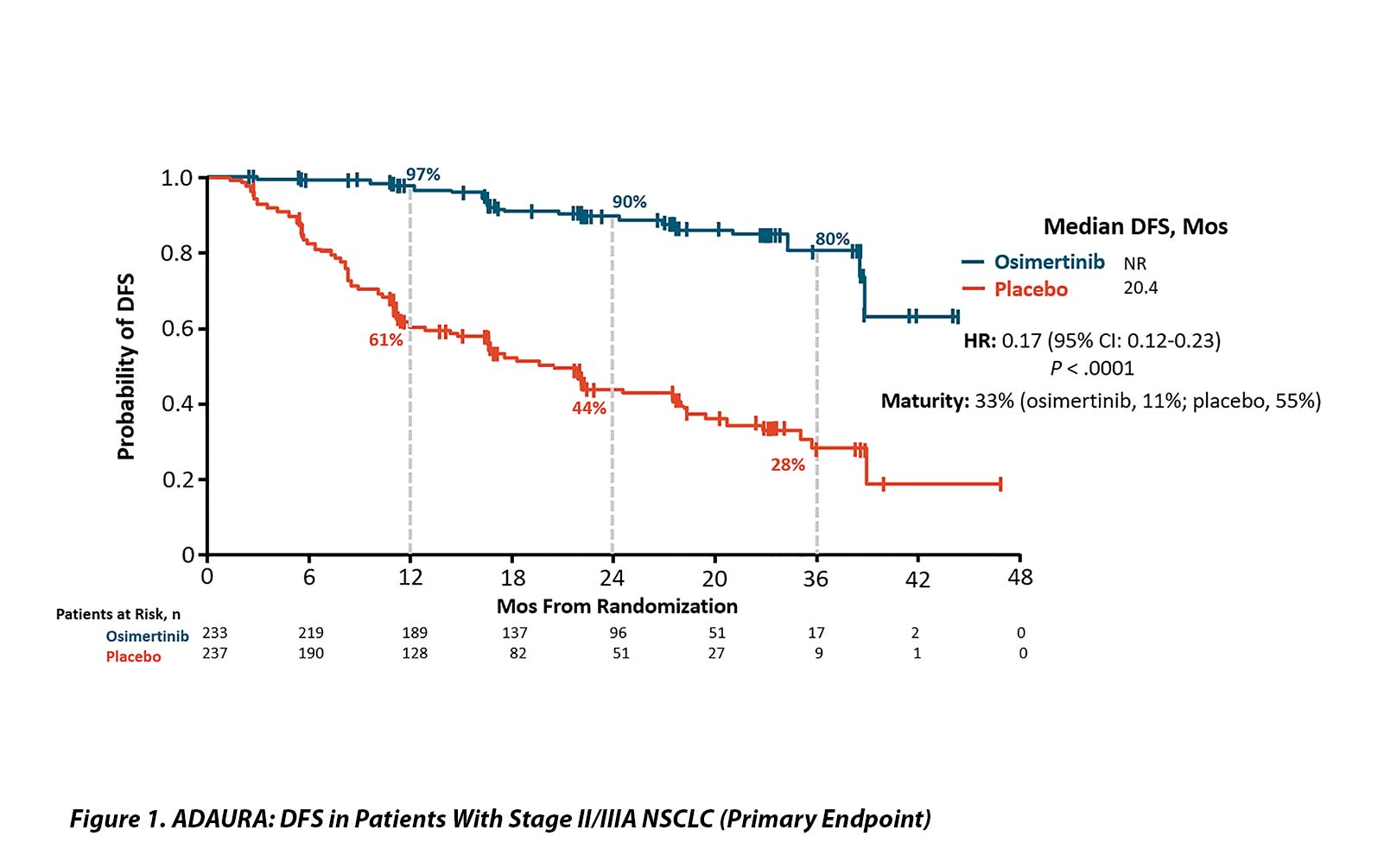
With 33% maturity, the DFS results were dramatic. The HR for patients with stage II and IIIA disease was 0.17 (95% CI [0.12, 0.23]; p < 0.0001) with median DFS of 20.4 months in the placebo group and median not reached in the osimertinib arm. The DFS efficacy appears stronger with increasing stage but remained highly statistically significant in the entire study population (HR 0.21 [0.16, 0.28]; p < 0.0001) and for the IB, II, and IIIA groups of patients (HR 0.50; 0.17 and 0.12, respectively). OS data remain extremely early with only 5% maturity and no obvious separation of the curves.
Toxicity data showed no unexpected safety signals for osimertinib and were quite similar to other published data (FLAURA). The discontinuation rate in the osimertinib arm was 11% compared with 4% in placebo, and adverse events leading to dose reductions occurred in 7% in the osimertinib arm versus 1% in the placebo control. Grade 3 and higher toxicity was seen in 10% of the patients treated with osimertinib and in 3% of patients receiving placebo.
ADAURA needs to be placed in context. The Chinese Thoracic Oncology Group (CTONG) 1104 investigators updated their post-operative gefitinib results at the ASCO20 Virtual Scientific Program as well.6,7 The CTONG trial had a unique design and compared 2 years of post-operative gefitinib to cisplatin-vinorelbine in a nearly identical setting. Despite a significant improvement in DFS, gefitinib failed to yield an OS benefit. It remains to be seen if the DFS benefit seen with osimertinib will translate into an OS benefit in ADAURA. OS is a hugely important secondary endpoint in this trial that will be long in coming and potentially affected by subsequent therapies for patients who experience relapse of their lung cancer, including the opportunity to cross over to osimertinib upon progression as part of the ADAURA design. As patients remain blinded and on study, it is unclear what the influence of unblinding will have on patients’ decisions to switch to active drug if regulatory approvals are granted for post-operative osimertinib. Other endpoints that may shed light on which patients are most likely to benefit from adjuvant osimertinib include circulating biomarkers as measures of residual disease. Finally, it will be critically important to follow the patients in the ADAURA trial for quality of life data and emerging sites of metastases, given the known superiority of central nervous system penetration for osimertinib compared with gefitinib.7 Ultimately, the patient perspective will be invaluable in interpreting the ADAURA results and will likely prompt thorough discussions for future patients with EGFR-mutated resected NSCLC. ✦
About the Author: Dr. Fidler is an associate professor in the Department of Internal Medicine at Rush Medical College. She is a member of the IASLC Communications Committee and the ILCN Editorial Group.
References:
1. Herbst RS Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB– IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol. 2020;38 (suppl; abstr LBA5).
2. Janjigian YY, Park BJ, Zakowski MF, et al. Impact on Disease-Free Survival of Adjuvant Erlotinib or Gefitinib in Patients With Resected Lung Adenocarcinomas That Harbor EGFR Mutations. J Thorac Oncol. 2011;6(3):569-575.
3. Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol. 2014;32:5s, (suppl; abstr 7514).
4. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib Versus Placebo in Completely Resected Non-Small-Cell Lung Cancer: Results of the NCIC CTG BR19 Study. J Clin Oncol. 2013;31(27):3320-3326.
5. Kelly K, Altorki NK, Eberhardt WEE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2015;33(34):4007- 4014.
6. Zhong W-Z, Wang Q, Mao W-M, et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFRmutant NSCLC (ADJUVANT/CTONG1104): A Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2018;19(1):139-148.
7. Wu YL, Shong W-Z, W ang Q, et al. CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation— Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J Clin Oncol.2020;38(suppl; abstr 9005).