Long-term follow-up data from a phase 1 CHRYSALIS trial cohort confirms amivantamab’s efficacy for select advanced non-small cell lung cancer (NSCLC) patients whose disease has progressed on previous lines of therapy—without additional safety concerns.
The data, presented by Pilar Garrido, MD, PhD, on March 29 during the 2023 European Lung Cancer Congress in Copenhagen, builds on CHRYSALIS results initially presented at the 2020 World Conference on Lung Cancer. The CHRYSALIS study evaluated amivantamab monotherapy in advanced NSCLC patients with EGFR exon 20 insertion mutations who experienced disease progression on platinum-based chemotherapy. The initial results showed an overall response rate (ORR) of 40% with an average duration of response of 11.1 months.
Dr. Garrido, who is head of the thoracic tumors section in the medical oncology department at University Hospital Ramón y Cajal, Madrid, said a total of 114 heavily pretreated patients were included in the follow-up analysis.
Treatment Benefits
“In terms of the efficacy, as of the data cutoff of 12 September 2022, the response rate was 37% and the median duration of response was 12.5 months,” she said. “Considering that more than 40% of patients had received prior immunotherapy and 20% of patients had received a prior EGFR TKI, amivantamab also demonstrated consistent efficacy regardless of prior therapy type.”
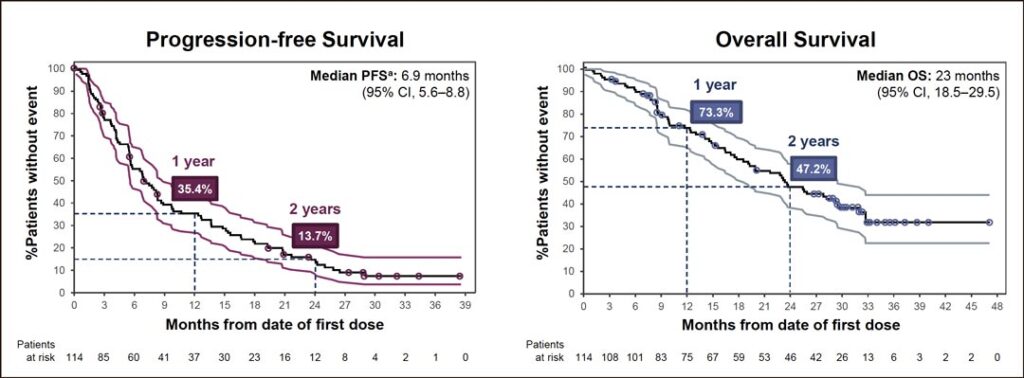
Dr. Garrido said there were no significant differences in efficacy in the subgroup analysis by age, sex, race, baseline ECOG status, number of prior lines of treatment, smoking status, or baseline brain metastasis status. She shared Kaplan-Meier curves showing progression free survival (PFS) and overall survival (OS).
“After a median of two prior lines of therapy and a median follow-up of 19.2 months, the median overall survival of amivantamab was 23 months, with a 2-year landmark OS rate of 47%,” Dr. Garrido said. “Forty-eight patients—42%—had sustained clinical benefit, meaning that they received amivantamab for at least 12 cycles. In fact, at the data cutoff, treatment was ongoing in 15 out of 114 patients. That represents 13% of patients who receive amivantamab for a median 2.6 years. Of those patients, seven are progression-free and eight are receiving treatment beyond progression.”
Predictors of Response
Dr. Garrido also reviewed potential predictors of sustained clinical benefit.
“In the univariate analysis, we considered age, sex, race, BMI category, ECOG status, brain metastasis status, smoker status, and median prior number of lines, but only ECOG status was associated with substantial clinical benefit,” she said. “In terms of potential predictive biomarkers, all patients gave a baseline plasma sample for ctDNA analysis. Absence of baseline RAS/RAF/MEK alterations was also associated with sustained clinical benefit.”
Safety
“In terms of safety profile, I think the first important message is no new safety signals were detected after long-term follow up,” Dr. Garrido said.
Rash was the most common adverse event and was experienced by 89% of patients with EGFR exon 20 insertions. Infusion-related reactions mostly occurred during the first cycle, and most of these were grade 1 and 2, she said.
In terms of treatment-related dose interruptions, reductions, and discontinuations, Dr. Garrido said 29% of patients had dose interruptions, 18% of patients had dose reductions, and 7% of patients required discontinuation.