The randomized phase III ADAURA trial stirred up considerable excitement last year when results from an unplanned interim analysis revealed an 80% reduction in the risk of disease recurrence or death with use of adjuvant osimertinib in comparison with placebo among patients with stage IB to IIIA NSCLC, far exceeding expectations for the global trial.1 Although the initial data readout for ADAURA was not planned until February 2022, the efficacy signal for osimertinib was so strong that the Independent Data Monitoring Committee recommended early unblinding of the results.
Osimertinib was subsequently approved by the US Food and Drug Administration in late December for use in the adjuvant setting among patients with EGFR mutation-positive NSCLC, even though the disease-free survival (DFS) data on which the approval was based had reached only 29% maturity at the time of analysis. Notably, only 5% of the events needed to trigger an analysis of overall survival (OS) had occurred, leaving open the possibility that the DFS results will not translate into an OS benefit.
New research presented at the 2020 World Conference on Lung Cancer bolster the initial ADAURA results, providing insight into patient health-related qualify of life (HRQoL) and the degree of DFS benefit according to the use of adjuvant chemotherapy.
Previously Published Results
Before diving into the new data, it is important to review the published ADAURA data that led to adjuvant osimertinib approval.
ADAURA included 682 patients with completely resected stage IB, II, or IIIA nonsquamous NSCLC that harbored either the exon 19 deletion or the L858R mutation in EGFR.1 Radiation therapy was not permitted, but patients could receive adjuvant chemotherapy per physician or patient choice. Following the completion of standard treatment, patients were randomly assigned to receive osimertinib or placebo for 3 years or until disease recurrence or fulfillment of a criterion for discontinuation.
The primary endpoint of interest was DFS in patients with stage II to IIIA disease, for which a hazard ratio (HR) of 0.70 was predicted. As it turned out, the HR was 0.17 (99.06% CI 0.11-0.26; P < 0.001), corresponding to an 83% reduction in the risk of disease recurrence or death with use of adjuvant osimertinib in comparison with placebo among patients with stage II to IIIA disease.
These findings remained consistent for the overall population of patients with stage IB to IIIA disease, where the HR with osimertinib versus placebo was 0.20 (99.12% CI 0.14-0.30; P < 0.001).
As mentioned, the OS data were too immature to estimate survival outcomes. At the time of data cutoff, 29 patients had died, 9 in the osimertinib group and 20 in the placebo group.
In terms of toxicity, osimertinib was associated with higher rates of grade 3 or greater adverse events (20% vs. 13%), dose interruptions due to adverse events (24% vs. 11%), dose reductions due to adverse events (9% vs. 1%), and drug discontinuation due to adverse events (11% vs. 3%) when compared with placebo. The most common all-grade adverse events that occurred more frequently with osimertinib than placebo included diarrhea (46% vs. 20%), paronychia (25% vs. 1%), dry skin (23% vs. 6%), pruritus (19% vs. 9%), and stomatitis (18% vs. 4%).
New Results on HRQoL
A key aspect of any cancer treatment is to achieve clinical benefits without compromising patient quality of life due to treatment toxicity, inconvenience, or some other factor. “The effect of adjuvant treatment on HRQoL is an important clinical consideration for patients who are disease free and require long-term treatment to reduce the risk of recurrence,” stated Margarita Majem, MD, PhD, of the Hospital de la Santa Creu i Sant Pau in Barcelona, Spain.
A key secondary endpoint of ADAURA was HRQoL as measured by the SF-36 instrument. Dr. Majem, who presented the HRQoL data (Abstract OA06.03), explained that this non-cancer–specific patient-reported questionnaire was considered appropriate for ADAURA since patients were cancer-free after surgery and since the tool has been used in other adjuvant therapy trials.
Patients completed the SF-36 questionnaire at baseline (randomization), 12 weeks, 24 weeks, then every 24 weeks thereafter until disease recurrence, treatment completion, or discontinuation (whichever occurred first). Outcomes were evaluated for the eight individual SF-36 health domains, as well as for two aggregated summary scores, the Physical Component Summary (PCS) and the Mental Component Summary (MCS). Patient compliance in completing the SF-36 remained high throughout the study, never dropping below 85% in either arm.
Dr. Majem showed data illustrating that the baseline PCS and MCS T-scores were similar for the osimertinib and placebo arms at baseline, all ranging from 46 to 47—values only slightly lower than the average seen in the general population (ie, 50). Very similar scores between the two arms were also observed across the 8 individual SF-36 health domains.
Importantly, the data showed that SF-36 scores were maintained over time from baseline through Week 96, with no clinically meaningful differences observed between arms with regard to scores for PCS, MCS, and the individual health domains (Fig 1).
Dr. Majem also reported that more than 80% of patients in each arm did not experience a clinically meaningful deterioration in PCS or MCS. Among those who did, there were no major differences in the time to deterioration between the osimertinib arm and the placebo arm in the PCS (HR 1.17, 95% CI 0.82-1.67) or the MCS (HR 0.98, 95% CI 0.70-1.39). These trends also persisted for all of the individual domains.
“These data indicate that HRQoL was maintained during adjuvant osimertinib treatment, with no clinically meaningful differences versus placebo. Coupled with a significant DFS benefit observed in these completely resected patients with stage IB to IIIA EGFR-mutated NSCLC. These data further support adjuvant osimertinib as an effective new treatment strategy in this setting,” Dr. Majem concluded.
Adjuvant Chemotherapy Use and Outcomes
Adjuvant cisplatin-based chemotherapy is recommended for patients with high-risk stage IB, stage II, and stage IIIA disease to minimize disease recurrence, and observational studies suggest that 48% to 57% of these patients receive such treatment in real-world practice.2,3 A key feature of the ADAURA trial was that patients could receive standard postoperative adjuvant chemotherapy prior to randomization based on physician and patient preference.
As reported by Yi-long Wu, MD, of the Guangdong Lung Cancer Institute in Guangzhou, China, and co-chair of the World Conference on Lung Cancer, 60% of patients in ADAURA received adjuvant chemotherapy, which, per protocol, should have consisted of up to 4 cycles of a platinum-based doublet (Abstract OA06.04). Adjuvant chemotherapy use was more common in individuals with stage II or IIIA disease versus stage IB disease (76% vs 26%), patients younger than 70 years of age versus 70 and above (66% vs 42%), and patients enrolled in Asia versus elsewhere (65% vs 53%).
Importantly, DFS was consistently better with osimertinib versus placebo regardless of whether patients received adjuvant chemotherapy. Among patients who received chemotherapy following resection, median DFS was not reached in the osimertinib arm but was 22.1 months in the placebo arm (HR 0.16, 95% CI 0.10-0.26). Similarly, among patients who did not receive chemotherapy following resection, median DFS again was not reached with osimertinib but was 33.1 months with placebo (HR 0.23, 95% CI 0.13-0.40).
“In the overall population, higher disease recurrence rates observed among patients in the placebo arm who received adjuvant chemotherapy compared with those who did not were likely driven by the large proportion of patients with stage II/IIIA disease,” Dr. Wu explained.
DFS outcomes clearly favored patients who received osimertinib as opposed to placebo across all disease stages regardless of whether or not patients also received adjuvant chemotherapy. Across all these subgroups, DFS HRs ranged from 0.10 to 0.38 (Fig 2).
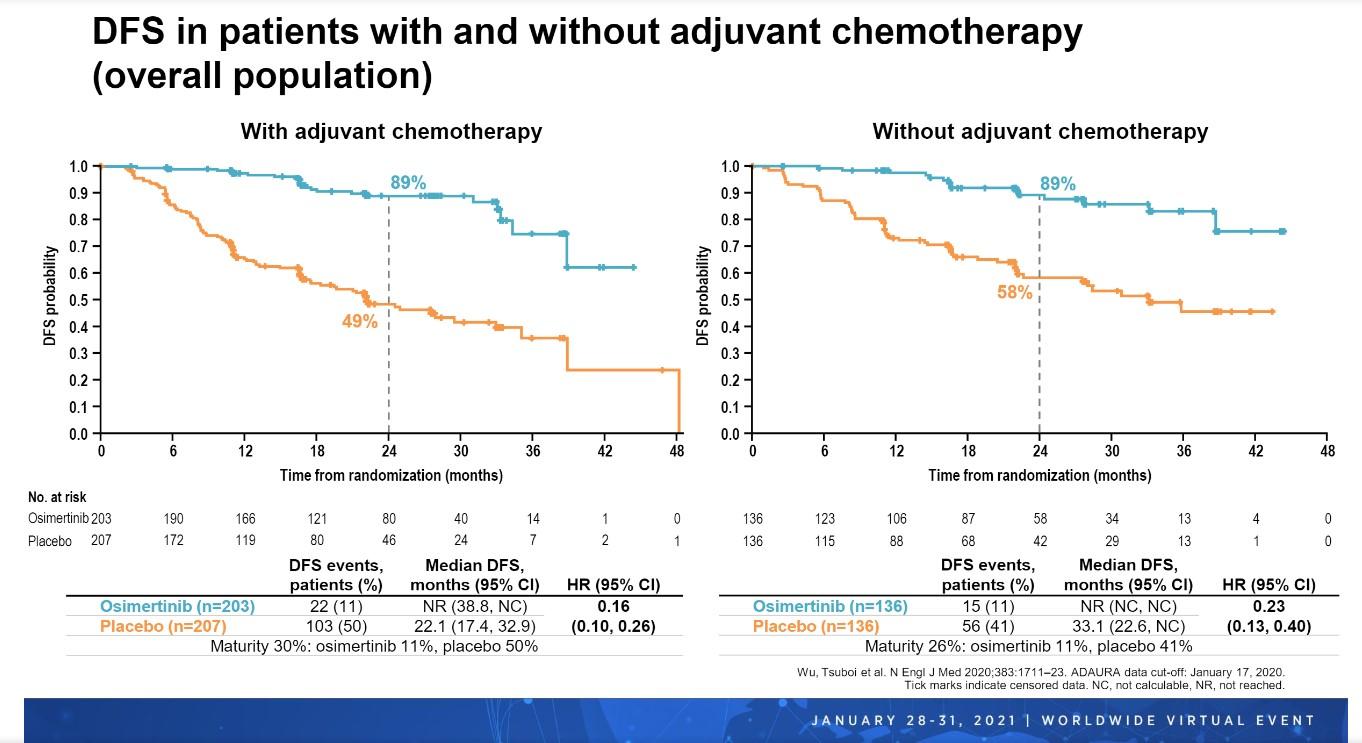
“DFS benefit with osimertinib versus placebo was observed irrespective of prior chemotherapy use or disease stage, supporting that adjuvant osimertinib will provide a highly effective treatment for patients with stage IB, II, or IIIA EGFR-mutated NSCLC after resection, with or without adjuvant chemotherapy,” Dr. Wu concluded.
Questions Answered, Questions Remaining
Haiquan Chen, MD, of the Fudan University Shanghai Cancer Center in China provided his thoughts on the two ADAURA abstracts presented.
He emphasized that the goal of any treatment is to enable patients to live longer and to live better. The results presented by Dr. Majem, which showed that adjuvant osimertinib did not affect HRQoL in patients with completely resected stage IB to IIIA EGFR-mutated NSCLC, “support the safety and efficacy of osimertinib in the adjuvant setting” and do not detract from the ability of patients to “live better,” according to Dr. Chen.
Dr. Chen felt that results presented by Dr. Wu are important because they support the application of osimertinib in patients who receive adjuvant chemotherapy as well as those who do not. Even “in the era of targeted therapy and immunotherapy, chemotherapy, we believe, still plays an important role in the treatment of NSCLC,” stated Dr. Chen, underscoring the importance of Dr. Wu’s analysis.
Despite these new ADAURA findings, questions still remain for Dr. Chen. First, should chemotherapy or an EGFR tyrosine kinase inhibitor be administered first in the adjuvant setting to lead to better survival outcomes? Second, might a panel of biomarkers better select patients likely to benefit from an EGFR tyrosine kinase inhibitor, as opposed to just looking at EGFR mutations? And finally, since OS is still the gold-standard outcome, will the DFS advantage turn into an OS benefit? Only time will tell.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 60 days at wclc2020.iaslc.org.
References
- Wu YL, Tsuboi M, He J, et al; ADAURA Investigators. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711-1723.
- Chouaid C, Danson S, Andreas S, et al. Adjuvant treatment patterns and outcomes in patients with stage IB-IIIA non-small cell lung cancer in France, Germany, and the United Kingdom based on the LuCaBIS burden of illness study. Lung Cancer. 2018;124:310-316.
- Buck PO, Saverno KR, Miller PJ, Arondekar B, Walker MS. Treatment patterns and health resource utilization among patients diagnosed with early stage resected non-small cell lung cancer at US community oncology practices. Clin Lung Cancer. 2015;16(6):486-495.










