According to newly reported data from the ADAURA trial, treatment with osimertinib for up to three years following surgery significantly lowered the risk of death in patients with completely resected EGFR-mutated (EGFRm) non-small cell lung cancer.
These new data were presented June 4 by principal investigator Roy S. Herbst, MD, PhD, during the 2023 American Society of Clinical Oncology Annual Meeting.
Previously reported ADAURA data demonstrated osimertinib improved disease-free survival (DFS) and reduced the risk of metastases. However, Dr. Herbst, who is assistant dean for translational research at Yale School of Medicine, New Haven, Connecticut, said these previous positive results had not led to universal acceptance of osimertinib as standard of care in this patient population.
“Despite having these data and this drug being approved in many countries, overall survival is considered the gold standard for treatment efficacy, and everyone has been keenly awaiting these data,” Dr. Herbst said. “There are some places that have not approved it yet. There are still some physicians, many surgeons—even some of my surgical colleagues at Yale—who do not recommend osimertinib because they’re waiting to see if it improves survival.”
The data show it does.
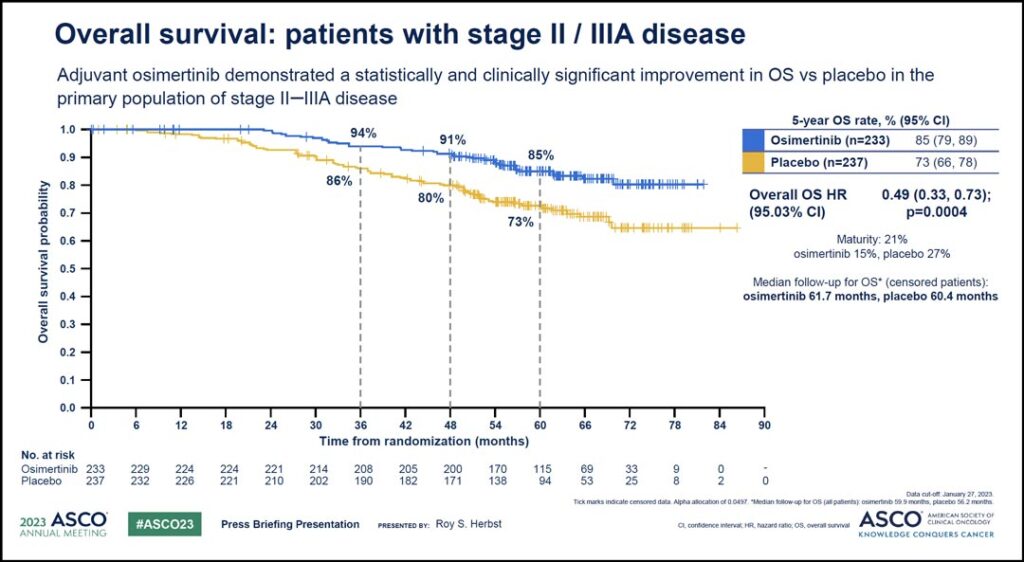
According to the findings, osimertinib demonstrated superior overall survival (OS) versus placebo in the study’s primary population, which included patients with stage II-IIIA disease, with an impressive hazard ratio of 0.49 equating to a 51% reduction in the risk of death. The 5-year overall survival rate with osimertinib was 85% compared with 73% with placebo.
“This result was highly statistically significant with a p value of 0.0004,” Dr. Herbst said. “The Kaplan Meier curves (see Fig. 1) show very early separation and sustained benefit beyond 5 years.”
Median follow-up was more than 5 years in both arms, and median overall survival was not reached in either treatment arm.
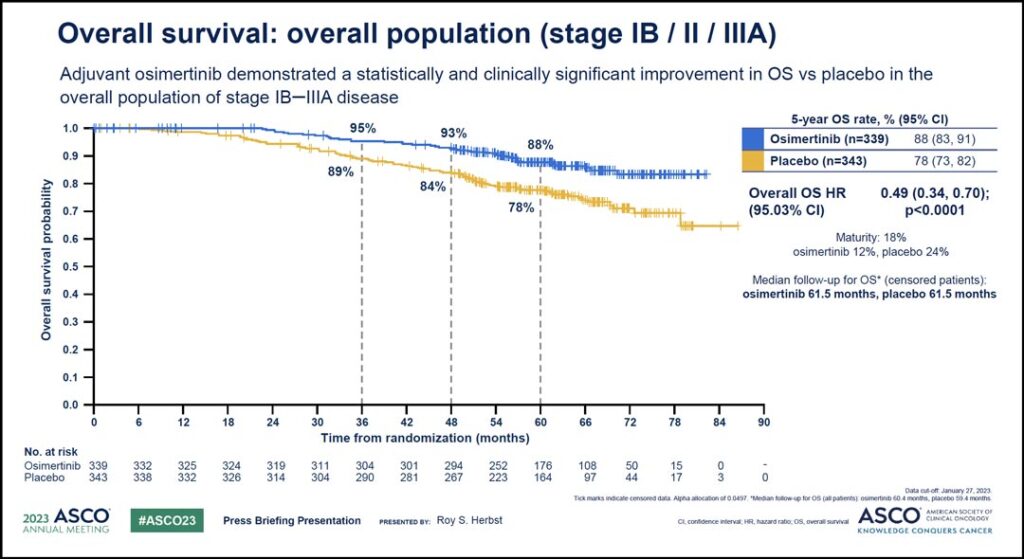
Similar positive results were seen in the analysis of the overall study population, which included patients with stage IB disease as well as patients with stage II-IIIA disease. (See Fig. 2) Again, the hazard ratio of 0.49 translated to a 51% reduction in the risk of death. The 5-year overall survival for these patients was 88% with osimertinib compared to 78% with placebo and the median follow-up for OS in all patients was 60.4 months with osimertinib and 59.4 months with placebo.
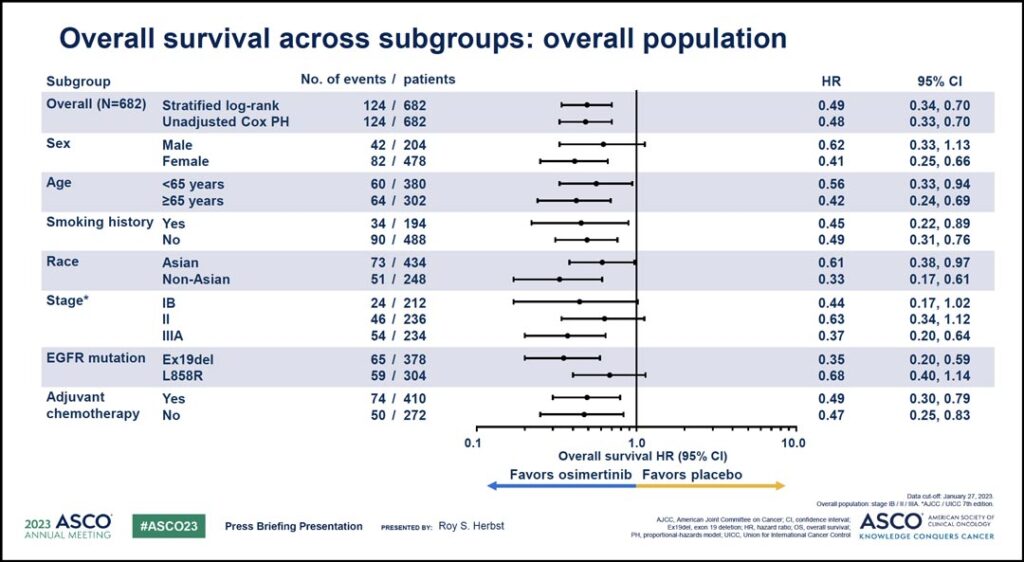
Benefit was seen in patients across a variety of subgroups.
“An exploratory analysis of overall survival in predefined subgroups demonstrated that osimertinib treatment led to consistent benefit across all patient subgroups with impressive hazard ratios ranging from 0.33 to 0.68,” Dr. Herbst said. “A clear clinical benefit was apparent regardless of stage, EGFR mutation type, race, and adjuvant chemotherapy use.” (See Fig. 3)
In ADAURA, use of adjuvant chemotherapy before randomization was allowed but not mandatory. Dr. Herbst said adjuvant chemotherapy was received by 60% of patients with an equal distribution of use across both treatment arms, and OS benefit with osimertinib was apparent regardless of prior adjuvant chemotherapy use.
Additionally, there were no new safety concerns reported as all patients had completed or discontinued study treatment as of the final analysis of previously reported DFS data.
Dr. Herbst said adverse events of grade 3 or greater were reported in 23% of patients in the osimertinib arm and 14% of the patients in the placebo arm. He said there were no treatment-related deaths in either arm at the time of this current data cutoff for overall survival. Only one serious adverse event was reported within 20 days after treatment discontinuation. This was a diagnosis of COVID-19 pneumonia, which was not treatment-related.
In all, these findings make ADAURA the first global phase III study to demonstrate statistically significant and clinically meaningful DFS and OS benefit with targeted treatment in this patient population.
“With the ADAURA DFS analysis from 3 years ago, we have precision medicine—the right drug for the right patient—to prevent metastasis and improve disease-free survival, and now we have improved overall survival,” Dr. Herbst said. “These findings reinforce adjuvant osimertinib as the standard of care for patients with resected, EGFR-mutated stage IB-IIIA non-small cell lung cancer and highlight the importance of screening and need for EGFR mutation testing as early as possible to broaden treatment access for patients.”
In response to the data, ILCN Editor Corey J. Langer, MD, FACP, said he was particularly impressed by the morphology of the survival curves, which showed further separation over time.
“I was not surprised to see improved overall survival based on the previously documented and profound DFS benefit, but I did not anticipate the degree of benefit beyond 4 and 5 years,” he said.