Surgical resection is the most effective treatment for patients with early-stage NSCLC.1
However, approximately 25% of these patients do not undergo resection, usually because of underlying comorbidities precluding surgery or because of patient refusal.2
For these patients and others with medically inoperable conditions, several guidelines recommend stereotactic body radiotherapy.3
However, the indication of stereotactic body radiotherapy is controversial in some populations; for example, interstitial lung disease or proximity critical mediastinal structures, such as major vessels and the heart, may increase the risks of radiotherapy ablation.
Alternative ablation techniques, including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, are promising treatments for malignant lung nodules. The first reported experience with lung tumor thermal ablation was an RFA case series4
in 2000. Further developments in RFA, MWA, and cryoablation have followed since. With the data available to date, it is not possible to conclude whether one modality is superior to another. Rather, each has its own advantages and disadvantages. RFA benefits from more than 20 years of experience with lung nodules, providing a more consistent understanding of its capabilities. A major limitation is its susceptibility to the heat-sink effect, whereby flowing blood within large vessels dissipates thermal energy.5
MWA can more rapidly increase tumor temperature than RFA and has less susceptibility to the heat-sink effect. However, experience with MWA is much more limited, and whether these differences have any clinical impact is unclear. One randomized controlled trial found no significant difference in OS between RFA and MWA at 12-month follow-up.6
Cryoablation has the advantage of preserving the underlying collagenous architecture; reduced transmitted injury to adjacent healthy tissue may have benefits for tumors near the pleura or major airways. This comes at the cost of increased procedure time and potentially increased bleeding risk compared with hyperthermal ablation (RFA, MWA), which otherwise generate thermal coagulation effects.7
Lung tumor ablation using these technologies is, at present, primarily performed percutaneously under image guidance. CT and cone-beam CT (CBCT) guidance are currently the most robust methods for localizing the tumor and probe tip during a percutaneous approach. Employing a percutaneous technique provides an advantage in stabilizing the probe position through the cumulative resistance from puncturing the skin, chest muscle, and lung parenchyma. However, percutaneous approaches can be associated with high complication rates. The most common complication is pneumothorax from pleural puncture:8
,9
the frequency of patients requiring chest-tube placement or aspiration post-RFA ranges from 2% to 59%. Pulmonary hemorrhage is also a common complication,10
,11
with an incidence of 6% to 18%. This risk is increased in the setting of central tumors, given the greater amount of parenchyma, with associated vasculature, that must be traversed.11
This risk with percutaneous ablation mirrors the experience seen with percutaneous biopsy. As such, to overcome the high rate of complications of the percutaneous approach, transbronchial ablation strategies are being evaluated, with the hope of replicating the safety benefit seen with transbronchial biopsy.12
,13
,14
,15
,16
The transbronchial approach naturally has a lower risk of pneumothorax, as the ablation probe does not pass through the pleura. Although the clinical experience with transbronchial ablation is limited, preliminary evidence suggests it has a more favorable safety profile compared with percutaneous ablation.14
,15
Similarly, the preliminary efficacy data are promising. A recent retrospective study presented by Chan et al.17
at the European Lung Cancer Virtual Congress 2021 summarized their experience with transbronchial MWA using electromagnetic navigation and CBCT confirmation for 41 nodules that had feeding bronchi into the targets (i.e., positive bronchus sign).17
At 1-year follow-up, disease control was achieved with all nodules.
A challenge with transbronchial techniques is the increased difficulty in accessing peripheral nodules. To improve nodule access and reduce procedure time, navigation systems such as electromagnetic navigation or virtual navigation may be helpful. CT and CBCT will likely be the more important technology, given their ability to provide immediate feedback on the probe position relative to the lesion. Obtaining and maintaining optimal probe position relative to the tumors is critical for effective ablation, including an appropriate margin. The recent development of robotic bronchoscopy may further improve the ability to precisely approach peripheral targets18
,19
with enhanced scope stability20
when integrated with ablation devices.
A major challenge with the transbronchial approach is that its effectiveness depends on the presence or absence of bronchi leading to the lung nodule. Targets without an accessible feeding bronchus require large ablation areas to ensure the nodule is included within the ablation field. One possible solution, proposed by Safi and colleagues,16
is employing bronchoscopic transparenchymal nodule access devices, currently being developed for nodule biopsy, to reach nodules independent of the bronchus sign.
Integrating transbronchial ablation into routine practice will ultimately require addressing the following: (1) the safety of ablation when close to the pleura and/or major central structures (e.g., heart, large vessels, large bronchi); (2) how well probe position can be maintained throughout ablation, especially in the lower lung fields, which are much more affected by respiratory motion; and (3) the cost-effectiveness of transbronchial approaches, including long-term disease control and safety. The existing literature is insufficient to answer these questions, but it is clear that significant progress has been made over recent years. Continued focus on research and clinical trials evaluating the safety and feasibility of transbronchial ablation is needed.
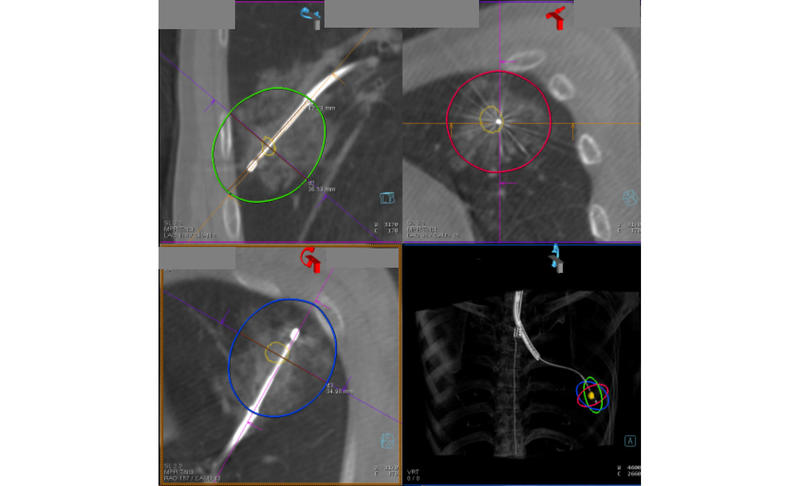
Fig. Transbronchial Microwave Ablation of Lung Cancer
- 1. McMurry TL, Shah PM, Samson P, et al. Treatment of stage I non-small cell lung cancer: What’s trending? J Thorac Cardiovasc Surg. 2017;154:1080-1087.
- 2. Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198-1205.
- 3. Videtic GM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7:295-301.
- 4. Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57-59.
- 5. Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101-111.
- 6. Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34:96.
- 7. Lubner MG, Hinshaw JL, Brace CL, et al. Cryoablation. In: Dupuy DE, Fong Y, McMullen WN, eds. Image-Guided Cancer Therapy: A Multidisciplinary Approach. Springer New York; 2013:61-78.
- 8. Rose SC, Thistlethwaite PA, Sewell PE, et al. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol. 2006;17:927-951; quiz 951.
- 9. Schneider T, Heussel CP, Herth FJ, et al. Thermal ablation of malignant lung tumors. Dtsch Arztebl Int. 2013;110:394-400.
- 10. Steinke K, King J, Glenn D, et al. Pulmonary hemorrhage during percutaneous radiofrequency ablation: a more frequent complication than assumed? Interact Cardiovasc Thorac Surg. 2003;2:462-465.
- 11. a. b. Nour-Eldin NE, Naguib NN, Mack M, et al. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur Radiol. 2011;21:197-204.
- 12. Tsushima K, Koizumi T, Tanabe T, et al. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J. 2007;29:1193-1200.
- 13. Suzuki H, Sekine Y, Saito K, et al. Innovative technique of transbronchial radiofrequency ablation for intrapulmonary tumors: a preliminary study in a rabbit model. J Bronchology Interv Pulmonol. 2011;18:211-217.
- 14. a. b. Koizumi T, Kobayashi T, Tanabe T, et al. Clinical experience of bronchoscopy-guided radiofrequency ablation for peripheral-type lung cancer. Case Rep Oncol Med. 2013;2013:515160.
- 15. a. b. Xie F, Zheng X, Xiao B, et al. Navigation Bronchoscopy-Guided Radiofrequency Ablation for Nonsurgical Peripheral Pulmonary Tumors. Respiration. 2017;94:293-298.
- 16. a. b. Safi S, Op den Winkel J, Kramer S, et al. A new bronchoscopic catheter for the transbronchial ablation of pulmonary nodules. Lung Cancer. 2018;124:125-129.
- 17. a. b. Chan J, Lau RW, Ng CS. Early experience of bronchoscopic transbronchial microwave ablation of lung nodules in the hybrid operating room. Abstract presented at: European Lung Cancer Virtual Congress 2021; March 25-27, 2021.
- 18. Yarmus L, Akulian J, Wahidi M, et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest. 2020;157:694-701.
- 19. Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest. 2021;159:845-852.
- 20. Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration. 2019;98:142-150.





