The novel antibody drug conjugate trastuzumab deruxtecan (T-DXd) demonstrated clinically meaningful and promising efficacy in patients with HER2-mutated NSCLC, according to interim results of the DESTINY-Lung01 trial, presented as part of the mini oral session, “MA11: Expanding Targetable Genetic Alerations in NSCLC.”
“These data demonstrate the potential of T-DXd as a new treatment option in patients with HER2-mutated NSCLC – a patient population with a high unmet need,” said Egbert F. Smit, MD, PhD, of Netherlands Cancer Institute, Amsterdam, who presented the results.
T-DXd is composed of an anti-HER2 antibody, cleavable tetrapeptide-based linker, and topoisomerase I inhibitor payload. A phase I trial of T-DXd in patients with HER2-mutated NSCLC showed objective response rate of 72.7%.
The ongoing, phase II DESTINY-Lung01 trial enrolled patients with non-squamous HER2-overexpressing or HER2-mutated metastatic NSCLC that was relapsed from or was refractory to standard treatment. Cohort 1 was HER2-overexpressing disease treated with T-DXd 5.4 mg/kg every 3 weeks. Cohort 2 was HER2-mutated disease treated with T-DXd 6.4 mg/kg every 3 weeks. The primary endpoint was confirmed overall response rate.
Data cutoff for the interim analysis was November 25, 2019. Dr. Smit presented results from Cohort 2 only. At the interim analysis, this cohort included 42 patients (64.3% female) with a median age of 63.0 years. A little less than one-half (45.2%) of patients had central nervous system metastases. The majority (90.5%) had prior platinum-based chemotherapy and approximately one-half (54.8%) had prior anti-PD-1/PD-L1 treatment. Median lines of prior therapy was two.
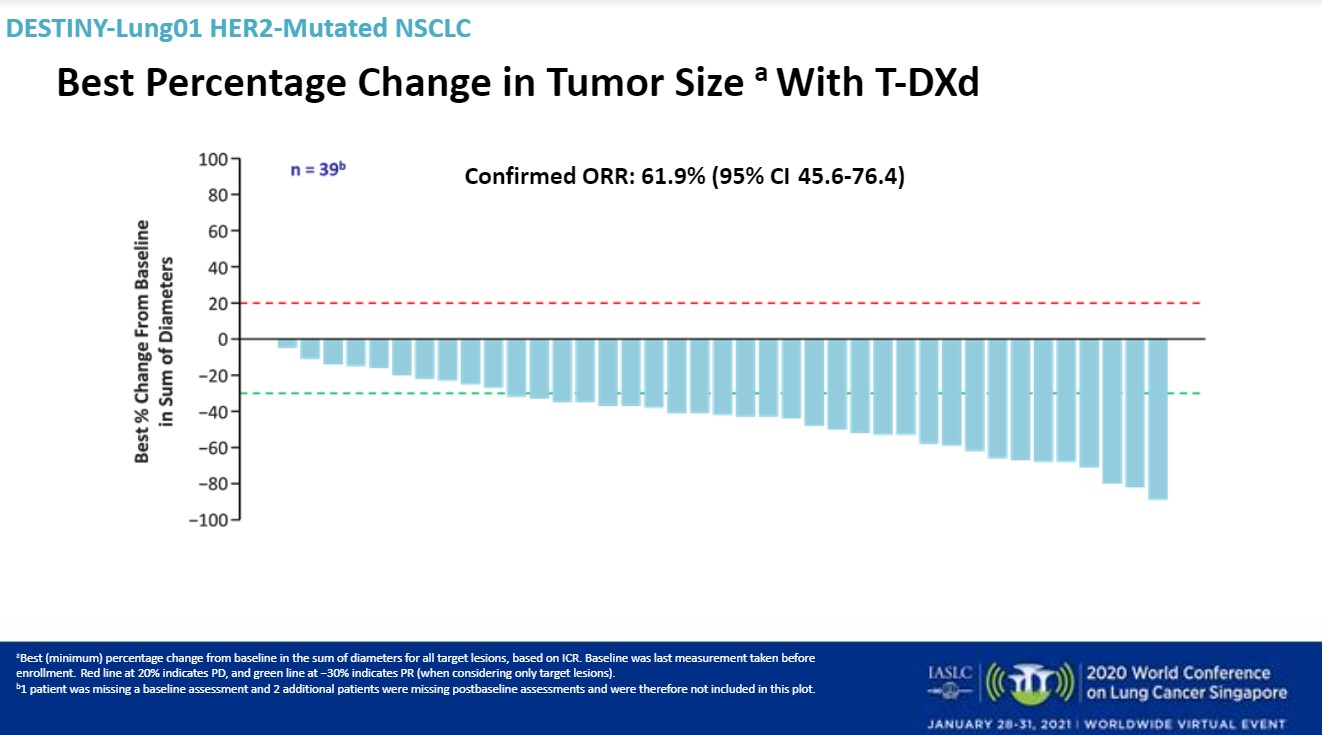
Median follow-up was 8 months. T-DXd 6.4 mg/kg demonstrated a high overall response rate and durable responses, Dr. Smit said. The confirmed overall response rate was 61.9% (95% CI: 45.6-76.4). There was one complete response and 25 partial responses.
The disease control rate was 90.5%, with a median duration of response not estimated. Sixteen of 26 responders remained on treatment. The median progression-free survival was estimated at 14 months. The median overall survival was could not be estimated.
The safety profile in the HER2-mutated cohort was generally consistent with what has been previously reported, Dr. Smit said.
The median treatment duration was 7.8 months. All patients had treatment-emergent adverse events; 64.3% were grade 3 or more, including decreased neutrophil count (26.2%) and anemia (16.7%).
More than one-half of these treatment-emergent adverse events (52.4%) were considered to be drug-related. Five patients had grade-5 treatment-emergent adverse events but none were judged to be related to treatment, Dr. Smit said. In addition, five patients had drug-related interstitial lung disease as adjudicated by an independent committee, but all were grade 2.
“Drug-related interstitial lung disease events observed in this patient population were low grade and there were no deaths,” Dr. Smit said. “However, interstitial lung disease remains an important identified risk for patients treated with T-DXd and requires careful monitoring and treatment.”
More than one-half (59.5%) of patients had treatment-emergent adverse events that led to dose interruption, and 38.1% had dose reduction. Treatment was permanently discontinued due to adverse events in 23.8% of patients.
Dr. Smit noted that enrollment in Cohort 2 was expanded with an additional 50 patients to better characterize T-DXd in patients with HER2-mutated NSCLC and further support the ongoing clinical trial program.
Study discussant Mark M. Awad, MD, PhD, Dana Farber Cancer Institute and Harvard Medical School, said that similar to EGFR exon 20 mutations HER2 activating mutations can also occur within exon 20, with a large number of insertional alterations.
Dr. Awad called the results of this study with T-DXd, a HER2 antibody–drug conjugate, impressive. In the DESTINY-Lung 01 trial patients had a response rate of about 62% and a median progression-free survival of 14 months. In terms of adverse events for T-DXd, there were a number of gastrointestinal side effects, some cytopenias and alopecia. However, the important side effect to note was interstitial lung disease, he said, which occurred in about 12% of this cohort. This is comparable to what was described in the DESTINY trials in breast and gastric HER2-positive cancers.
Ongoing priorities in this area will include further understanding of the mechanisms of resistance to antibody–drug conjugates and an understanding of who is at risk for developing toxicities. In addition, more research is needed on combination strategies to safely and effectively delay resistance.
The study was sponsored by Daiichi Sankyo.
References:
- Tsurutani J, Iwata H, Krop I, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discovery. 2020;doi:10.1158/2159-8290.CD-19-1014.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 60 days at wclc2020.iaslc.org.