Editor’s Note: ILCN’s editors say the definition of resectability needs to be revisited in the era of neoadjuvant immunochemotherapy. See From the Editors: Defining Resectability of Non-Small Cell Lung Cancer in 2023.

Thoracic oncologists following the ongoing debate about the pros and cons of neoadjuvant immune therapies for the treatment of resectable non-small cell lung cancer (NSCLC) have considerably more data to consider following the American Society of Clinical Oncology’s 2023 Annual Meeting, which took place June 2-6 in Chicago. During the meeting several investigators shared new findings from neoadjuvant IO studies incorporating IO with chemotherapy, including notable event-free survival (EFS) data from the KEYNOTE-671 and Neotorch studies.
Peri-operative Pembrolizumab Improves Event-Free Survival in KEYNOTE-671
Neoadjuvant pembrolizumab plus chemotherapy followed by surgery and adjuvant pembrolizumab provided statistically significant, clinically meaningful improvement in event-free survival (defined by local progression precluding definitive surgery, recurrence, or death) compared with neoadjuvant chemotherapy and surgery alone, according to findings from the KEYNOTE-671 trial.
The results were presented by the study’s lead author and IASLC President Heather Wakelee, MD.
KEYNOTE-671 is a randomized, double-blind, phase III study of pembrolizumab or placebo plus cisplatin-based chemotherapy followed by resection and pembrolizumab or placebo for resectable NSCLC.
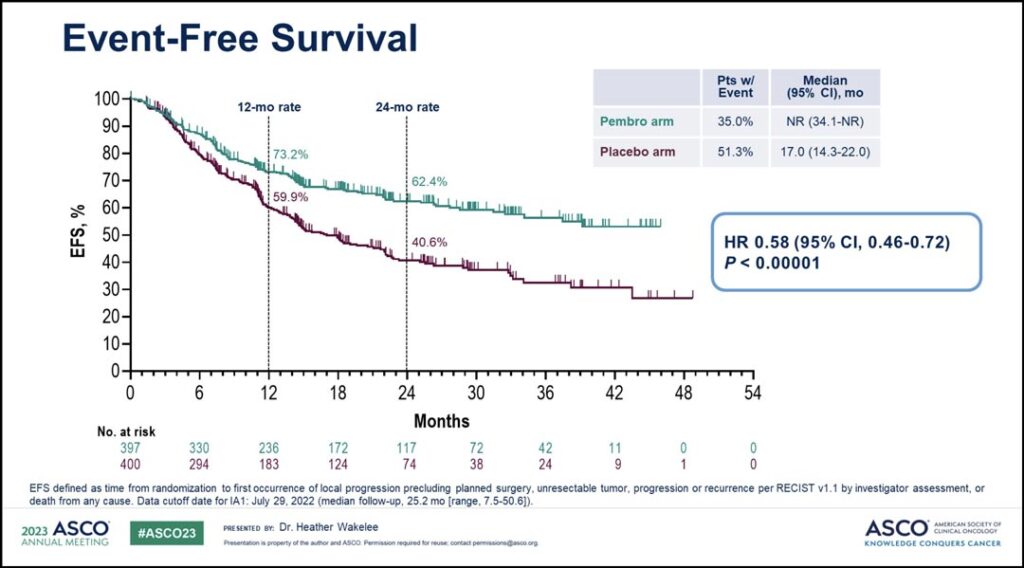
This study enrolled 797 patients. As of the July 29, 2022, the data cutoff, median follow-up was 25.2 months. EFS was significantly improved in the neoadjuvant pembrolizumab plus chemo arm compared to the placebo arm: not reached versus 17 months. (See Fig. 1)
Additionally, Dr. Wakelee said pathologic response rates were significantly higher in the pembrolizumab arm versus the placebo arm. The major pathologic response (mPR) rate was 30.2% versus 11% and the pathologic complete response (pCR) rate was 18.1% versus 4%. Exploratory analysis showed an EFS benefit for perioperative pembrolizumab regardless of whether patients achieved pCR or mPR.
And while the overall survival benefit of perioperative pembrolizumab had not reached statistical significance at the time of the data cut off, early data showed a separation in survival beyond two years, with a HR of 0.73 and p value of 0.02124. Though the significance boundary was not achieved at this early look, overall survival data will continue to be evaluated according to the statistical analysis plan, Dr. Wakelee said.
“These results indicate pembrolizumab given in combination with chemotherapy prior to surgery and for up to 1 year as a single agent after surgery led to significant improvement in event-free survival with a strong trend towards improvement in overall survival, whether or not patients achieved a pCR or major pathologic response.” she said. “These results indicate perioperative pembrolizumab significantly improves outcomes for patients with potentially resectable stage II-III NSCLC.”
Addition of Toripalimab Shows Promise in Interim Analysis of Phase III Neotorch Trial

Shanghai Jiao Tong University’s Shun Lu, MD, PhD, lead author of the Neotorch trial, shared detailed results from the interim analysis of the study—a randomized, double-blind, placebo-controlled phase III trial exploring the addition of perioperative toripalimab to chemotherapy for the treatment of resectable stage III NSCLC.
Dr. Lu said the addition of toripalimab to perioperative chemotherapy led to an improvement in event-free survival for patients with stage III resectable NSCLC.
“The Neotorch study is one of the first studies to present the efficacy and safety data of an anti-PD-1 antibody in early-stage NSCLC as perioperative treatment and to provide the evidence for the selection of dosage and treatment duration of anti-PD-1 antibody treatment,” Dr. Lu said. “These findings support establishing anti-PD-1 antibody treatment in combination with chemotherapy as the backbone regimen for patients with resectable NSCLC, which will change clinical practice in the near future.”
In the study, 404 patients with stage III NSCLC were randomized 1:1 to receive either the anti-PD-1 monoclonal antibody toripalimab or placebo in combination with chemotherapy every three weeks for three cycles before surgery and one cycle after surgery, followed by toripalimab or placebo monotherapy every three weeks for 13 cycles. After a median follow-up of 18.3 months, EFS had significantly improved in patients receiving toripalimab.
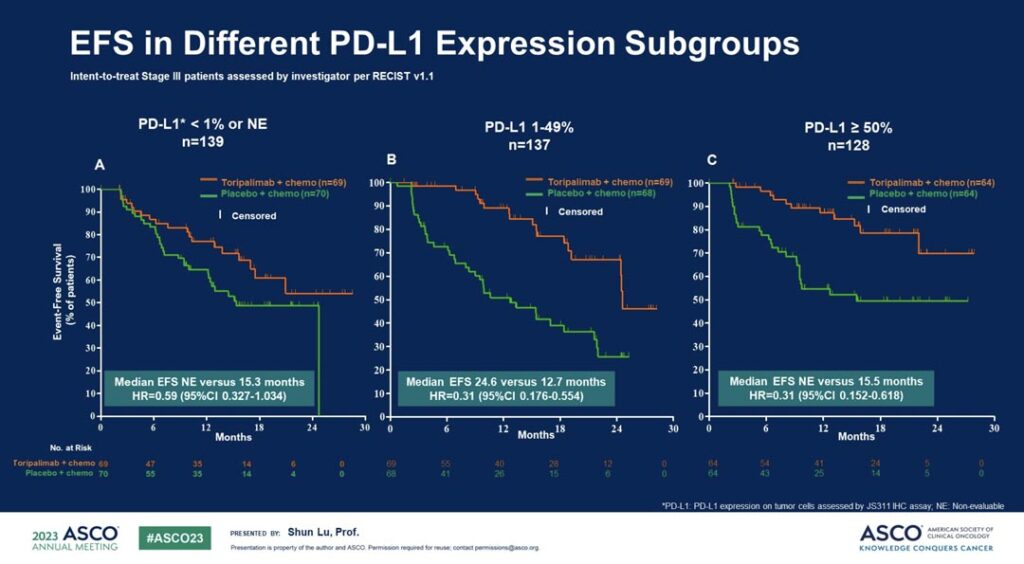
Dr. Lu said that while the EFS benefit was more prominent among patients with positive PD-L1 expression, all patients with NSCLC appeared to benefit from toripalimab treatment, regardless of PD-L1 expression status. (See Fig. 2)
The median EFS was not reached in the toripalimab arm and was 15.1 months in the placebo arm. Additionally, mPR and pCR rates were higher in the toripalimab arm compared to the placebo arm (48.5% vs 8.4% and 24.8% vs 1.0%, respectively).
Dr. Lu said the research team also observed a trend in overall survival results favoring toripalimab plus chemotherapy compared to placebo plus chemotherapy. Point estimate 2-year survival rate for the toripalimab arm was 81.2% versus 74.3% for the placebo control (HR 0.62, p = 0.052). Patients will continue to be followed for overall survival results.
ILCN Editor Corey J. Langer, MD, FACP, said he believes these are “breakthrough” trials with the potential to alter the therapeutic landscape in resectable, stage II-III NSCLC.
“We will ultimately need trials comparing peri-adjuvant strategies to adjuvant therapy alone, as well as further details regarding long-term outcomes in those with suboptimal responses and in those with residual N2 disease post resection,” Dr. Langer said.