Squamous cell lung cancer (SqCLC), which accounts for approximately 25% to 30% of all lung cancer cases, is the second-most prevalent histologic subtype of NSCLC. Its distinct clinicopathologic and molecular characteristics present obstacles to the development of treatments for SqCLC, resulting in a poorer prognosis. It affects patients who are older, who have more comorbidities or a history of heavy smoking. It presents at an advanced stage, proliferates rapidly, and almost always lacks known molecular drivers, with the result that most targeted therapies are not suitable for patients who have SqCLC. Furthermore, anti-angiogenesis agents such as bevacizumab are also not recommended because their administration increases the risk of hemorrhage. As a result, chemotherapy has historically remained the backbone of treatment, with a median OS of less than 1 year. It is therefore essential to develop novel therapeutic strategies for this specific population. Fortunately, immune checkpoint inhibitors (ICIs) have revolutionized the treatment paradigm of NSCLC, including SqCLC. Several phase III studies have already demonstrated the superiority of PD-1 inhibitors in combination with chemotherapy compared to chemotherapy alone as the front-line treatment of choice for advanced SqCLC; these include KEYNOTE-407,1
RATIONALE-307,2
,3
CameL-sq,4
and ORIENT-12.5
The phase III ORIENT-12 trial was conducted in 42 centers in China. A total of 357 participants with previously untreated stage IIIB-IV SqCLC were randomly assigned to groups in equal number to receive either the checkpoint inhibitor sintilimab or placebo in combination with a platinum-based chemotherapy drug plus gemcitabine (GP). The study resulted in a statistically significant improvement in the primary endpoint of independent radiographic review committee (IRRC)-assessed PFS. The median PFS was 5.5 months for patients who received sintilimab-GP versus 4.9 months for those who received placebo-GP (hazard ratio [HR] 0.536, 95% CI [0.422, 0.681]; p < 0.00001). Of note, the 12-month PFS rate was 22.3% in the sintilimab-GP group versus 3.1% in the placebo-GP group.
ORIENT-12 is the first randomized phase III study to use GP in combination with a PD-1 inhibitor in patients with advanced SqCLC, thus providing a new option for combination therapy in this population. Other key clinical studies in patients with SqCLC including KEYNOTE-407, RATIONALE-307, and CameL-sq instead used platinum-based chemotherapy with paclitaxel or nanoparticle albumin-bound paclitaxel (nab-paclitaxel) as the partner for immunotherapy, because the immunomodulatory mechanisms of paclitaxel and nab-paclitaxel are widely researched. On the one hand, paclitaxel can initiate immune response by increasing the rate of apoptosis in tumor cells, releasing tumor antigens, and enhancing the phagocytosis of antigen-presenting cells (APC). On the other hand, paclitaxel can reverse immunosuppression by reducing the number of regulatory T cells (Tregs) and inducing M1 tumor-associated macrophage (TAM) differentiation. Nab-paclitaxel has been developed to overcome severe allergic reactions, avoiding immune suppression by sparing glucocorticoid preconditioning, although there were no significant differences in PFS and OS between paclitaxel and nab-paclitaxel when each was used as a combination partner in subgroup analyses. However, because of the high frequency of alopecia and neuropathy associated with their use, many patients consider both of those drugs unacceptable. Therefore, the introduction of new chemotherapeutic partners for ICIs are needed, and the ORIENT-12 study has contributed to the expansion of the treatment landscape for SqCLC.
GP is also a standard chemotherapy regimen for SqCLC, most frequently used in China. A phase II study suggested that the efficacy of GP was not inferior to platinum/nab-paclitaxel as a first-line treatment for advanced SqCLC, and that the incidence of grade 3 or 4 neutropenia with this treatment was lower than that in patients who received carboplatin/nab-paclitaxel.6
Moreover, many recent studies in animal models have proven the immunomodulatory role of gemcitabine(Fig. 1). For example, Ho et al,7
observed increased infiltration of Th1 lymphocytes and M1 macrophages after treatment with gemcitabine plus an anti-PD-1 antibody in a pancreatic ductular adenocarcinoma (PDAC) murine model. Principe et al,8
also discovered that gemcitabine could sensitize PDAC tumors to ICIs by enhancing antigen presentation and increasing synthesis of pro-inflammatory chemokines. Notably, in a model of murine lung carcinoma, gemcitabine was found to induce tumor-cell apoptosis and PD-L1 expression and increase the proportion of CD8-positive and CD4-positive T cells, conferring a synergistic anti-tumor effect in combination with anti-PD-1 therapy.9
Fig. The Immunomodulatory Role of Gemcitabine in the Tumor Microenvironment Supports its Combination With Immune Checkpoint Inhibitors
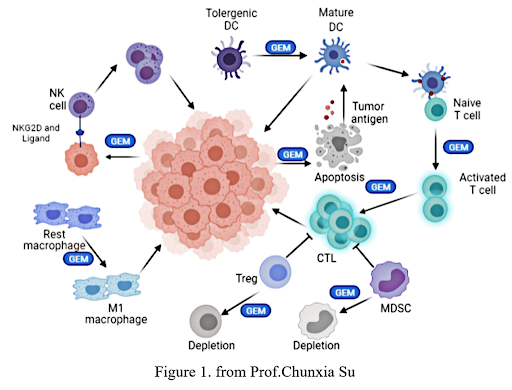
Abbreviations: CTL, cytotoxic T cell; DC, dendritic cell; GEM, gemcitabine; MDSC, myeloid-derived suppressor cell; NK cell, natural killer cell; NKG2D, an activating receptor of the NKG2 family; Treg, regulatory T cell.
Nevertheless, the immune effect that is commonly observed when different chemotherapy drugs are used in combination with ICIs still needs to be investigated broadly. There are some critical issues that should be taken into serious consideration when designing clinical studies. The first involves weighing, how to combine the specific drugs to maximize their efficacy. Differences in the interactions between chemotherapy agents and ICIs may lead to inconsistent efficacy and safety profiles. The interactions between the chemotherapy agents commonly used in clinical practice with PD-1/PD-L1 inhibitors need to be further investigated in preclinical models. Second, the majority of current combination therapies are administrated simultaneously. However, in theory, chemotherapy is more likely to induce an immune-supporting tumor microenvironment by releasing tumor antigen and allowing the immune system to recover before immunotherapy in a chemotherapy–immunotherapy combination regimen. Therefore, the complicated issue of treatment sequencing should be investigated in future trials. Third, the dose of the chemotherapy drug also matters. Is the full recommended dose and course of standard chemotherapy really required? As we know, chemotherapy has been developed on the basis of a maximum tolerated dosing philosophy, which is likely to directly kill immune cells and result in immunosuppression. For example, lessons from CheckMate-9LA suggest that a limited course of chemotherapy in combination with ICIs can prolong PFS and OS.10
Therefore, what might be the optimal dose and treatment course required to stimulate an adaptive immune response? Last, but not least, searching for biomarkers that predict response to combined chemotherapy and ICIs should be a vital focus of future studies. No single solution or study can provide all the needed guidance; instead, we will need to find the most likely beneficiaries of each specific treatment strategy.
In conclusion, the ORIENT-12 study supports the premise that combining sintilimab with GP as a first-line treatment for locally advanced or metastatic SqCLC is an innovative and feasible therapeutic option. However, there are still many challenges that will need to be resolved through future clinical studies, including optimal doses and combinations, the sequencing of various agents, and predictive biomarkers.
- 1. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2040-2051.
- 2. Wang J, Lu S, Yu X, et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7(5):709-717.
- 3. Wang J, Yu X, Lu S, et al. Phase III study of tislelizumab plus chemotherapy vs chemotherapy alone as first-line (1L) treatment for advanced squamous non-small cell lung cancer (sq NSCLC). J Clin Oncol. 2020;38(15).
- 4. Zhou C, Ren S, Chen J, et al. Camrelizumab or placebo plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A randomized, double-blind, multicenter, phase III trial. J Thor Oncol. 2021;16(4):S748-S748.
- 5. Zhou C, Wu L, Fan Y, et al. ORIENT-12: Sintilimab plus gemcitabine and platinum (GP) as first-line (1L) treatment for locally advanced or metastatic squamous non-small-cell lung cancer (sqNSCLC). Anna Oncol. 2020;31:S1186-S1186.
- 6. Wang Z, Huang C, Yang J-J, et al. A randomised phase II clinical trial of nab-paclitaxel and carboplatin compared with gemcitabine and carboplatin as first-line therapy in advanced squamous cell lung carcinoma (C-TONG1002). Eur J Cancer. 2019;109:183-191.
- 7. Ho TTB, Nasti A, Seki A, et al. Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis. J Immunother Cancer. 2020;8(2):e001367.
- 8. Principe DR, Narbutis M, Kumar S, et al. Long-Term Gemcitabine Treatment Reshapes the Pancreatic Tumor Microenvironment and Sensitizes Murine Carcinoma to Combination Immunotherapy. Cancer Res. 2020;80(15):3101-3115.
- 9. Du B, Wen X, Wang Y, Lin M, Lai J. Gemcitabine and checkpoint blockade exhibit synergistic anti-tumor effects in a model of murine lung carcinoma. Int Immunopharmacol. 2020;86:106694.
- 10. Du B, Wen X, Wang Y, Lin M, Lai J. Gemcitabine and checkpoint blockade exhibit synergistic anti-tumor effects in a model of murine lung carcinoma. Int Immunopharmacol. 2020;86:106694.






