Posted: October 2018
Patients with stage III or more advanced lung cancer tend to be older and less healthy than patients with other stage III cancers. Because of this, selection of optimal therapies for individual patients, including stereotactic body radiation therapy (SBRT), is more nuanced. With the advent of improvements in technology, more multidisciplinary approaches to decision making, and changing recommendations on fractionation, numerous factors influence radiation therapy selection and delivery often in the absence of an abundance of data. In addition, the rapid addition of immunotherapy in locally advanced NSCLC has resulted in even more questions and potential for rapid change in best practices.

In the following interview, Kristin Higgins, MD, associate professor and medical director of radiation oncology of The Emory Clinic at Winship Cancer Institute’s Clift on campus, explains her approaches to therapeutic decision making and provides an overview of the state of the art in radiation oncology technology.
Multidisciplinary Care for Patients with Early-Stage Disease
The current standard of care for early-stage NSCLC is surgical resection. However, many patients aren’t optimal surgical candidates, whether it’s because of damage to their lungs from years of smoking, risks associated with anesthesia, or potential perceived postoperative toxicities. These patients live in a gray zone of sorts. They are clearly not surgical candidates, and for them, the standard of care is sterotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR). There are often disagreements across subspecialties about nodule management for these patients because no trials that directly compare surgery with SBRT have met their accrual goals, many closing early or prematurely. The ongoing Veterans Aff airs Lung Cancer or Stereotactic Radiotherapy (VALOR) trial within the Veterans Affairs system is comparing SBRT with surgery for a high-risk population, but this is not open to patients who do not have a military service history.
In the meantime, we base our decisions for this high-risk population on the data we have available to us and on the best interest of the patient. More often, we’re trying to involve the patient in a multidisciplinary discussion that involves the surgeon, the radiation oncologist, and the medical oncologist so that the patient can hear the pros and cons for each potential treatment scenario and can participate in decision making. I think this shared approach is a good way to determine appropriate therapy for each individual patient when there is no black or white answer.
Treating Stage III Disease
The average age of a patient at lung cancer diagnosis is 70,1 which means that decisions regarding concurrent therapy should not be based solely on age. It’s important that decision making about combined modality treatment is a thoughtful process that involves geriatricians in the evaluation of candidacy, especially because the management of the side effects from combined-modality therapy has so drastically improved over time. If you look at RTOG 0617 for example, the rates of high-grade pneumonitis and esophagitis were only approximately 7% in the standard-dose arm,2 which was a decrease from the approximately 15% to 20% or higher rates observed in the first generation of combined modality trials for stage III lung cancer.3
There is great interest in immunotherapy and combination immunotherapy/radiation clinical trials for lung cancer, especially in the locally advanced setting. The standard of care has really shifted and now includes consolidated immunotherapy for stage III disease based on the positive PFS results of the phase III PACIFIC trial which, as presented at the 19th World Conference on Lung Cancer in September, also demonstrated a highly significant OS advantage. However, despite the emergence of immunotherapy in stage III NSCLC, a lot of questions remain. How do you approach immunotherapy in an elderly patient, for example, who may not be a candidate for combined- modality treatment? We’ve seen exciting results with immunotherapy given in the consolidative setting, but can it be moved into the concurrent setting? What is the optimal radiation dose/fractionation regimen to use with immunotherapy? There are developing clinical trials designed to answer these and other emerging questions around immunotherapy and locally advanced NSCLC.
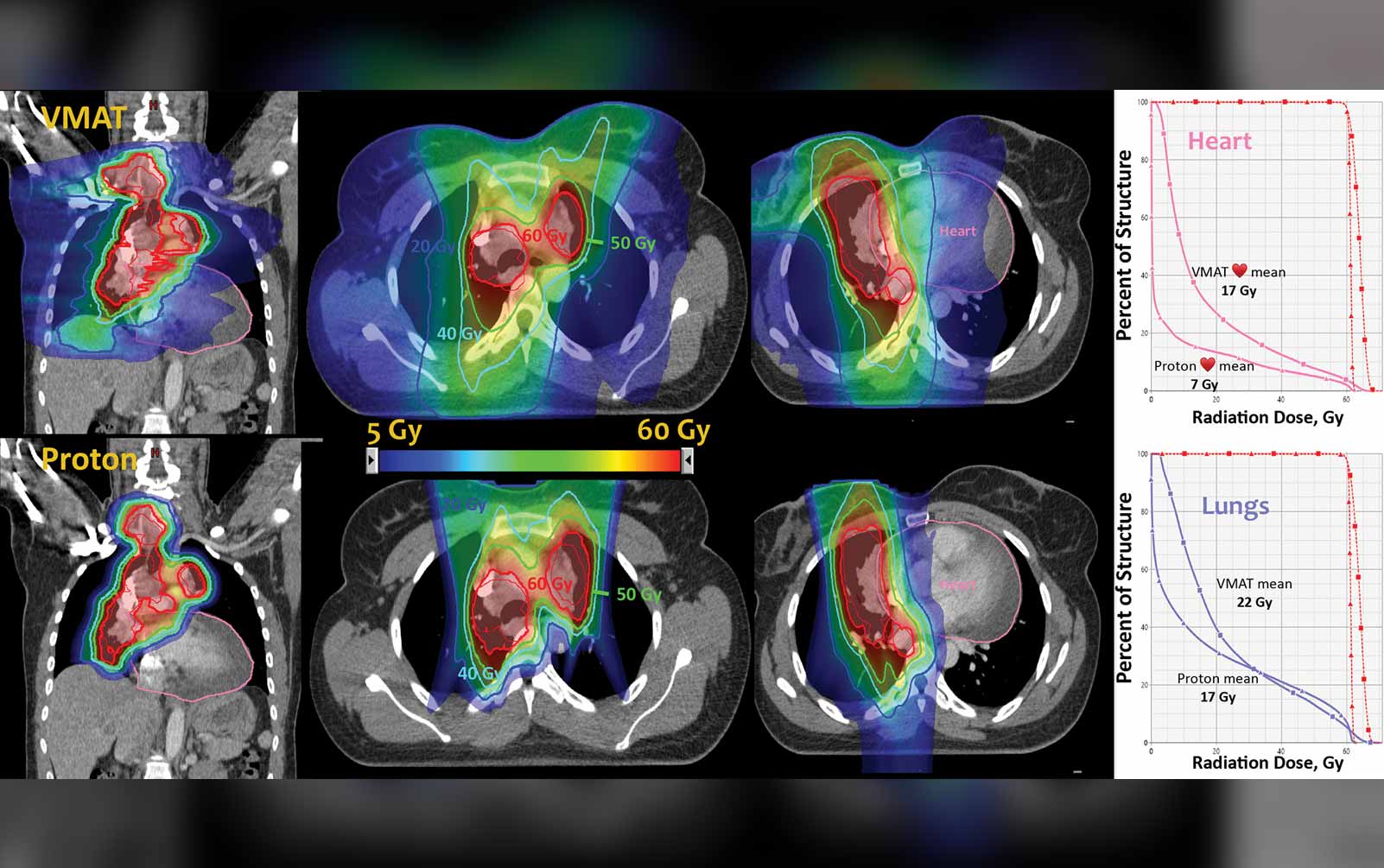
Proton Therapy’s Unproven Benefits
Proton therapy is being more widely used in the management of many cancers throughout the United States, with more and more proton centers coming online. The randomized phase III NRG 1308 trial (NCT01993810) is evaluating proton versus photon therapy for unresectable stage II and III NSCLC. The study design has recently been revised to include co-primary endpoints of overall survival, development of grade 2 or greater cardiac toxicity, and grade 4 or greater lymphopenias. RTOG 0617 demonstrated that a higher dose of radiation led to decreased survival. Importantly, this study also showed that when the radiation dose to the heart increased, there was a greater risk of mortality.2 With lung cancer, radiation dose to the heart is an obvious concern given the close proximity of lung tumors to cardiac structures. Using protons in stage III lung cancer makes a lot of sense from that standpoint because you can deliver an adequate dose to the tumor but decrease the bystander radiation dose to the heart, which cannot be done as well with standard photon techniques including intensity modulated radiation therapy (IMRT).
Until we see the results of this trial, however, I think that proton therapy should still be used wisely in patients in a clinical trial, which is really the best way to explore this technology.
Clinical Trials Versus the Real Word: Technology Must Be Biology Driven
In radiation oncology, we are technologically driven. We try to use our technologies to make our therapy more precise and accurate, but it is important to remember that these costly improvements must be clinically meaningful. Applications of technologies must lead to improvements in meaningful outcomes for our patients, such as reduced side effects, improved quality of life, and, of course, improved survival. To tackle some of these challenges, next-generation linear accelerators are being developed. They are more costly than standard linear accelerators, but they offer features such as built-in MRI, which allows tumor imaging in real time as the radiation beam is being directed at the tumor. One such accelerator, marketed by ViewRay, has been approved by the U.S. Food and Drug Administration (FDA). There’s also a next-generation machine, which is not yet FDA approved, that combines a linear accelerator with PET and can yield biologically guided radiation therapy, in which the photon beam is sent from the linear accelerator directly to the PET signal within the tumor.
The use of these next-generation machines could be advantageous in that you can potentially dose escalate tumors that are near critical organs because you can see the organs in real time and adapt the radiation to the exact anatomy of the patient at the time of treatment. Th is is especially helpful in pancreatic cancers, for example, and these machines are being used in prospective clinical trials. For lung cancer, this newer technology would potentially allow us to better target tumors during the respiratory cycle and to more safely dose escalate or treat multiple sites of metastatic disease simultaneously.
In addition to using trial data to prove that technologic improvements result in improved patient care, it is also important that we design radiation trials so that they are reflective of a real-world population. Future trials should be framed around the typical patient with lung cancer—elderly and often with a comorbid conditions, such as heart disease or diabetes—because, otherwise, we won’t be able to translate our findings from clinical trials with stricter inclusion criteria into real-world care.
Palliative Radiation SBRT
The American Society for Radiation Oncology consensus guideline for palliative thoracic radiation therapy for NSCLC recommends a longer course of 30-42 Gy delivered at 2.8-3 Gy per day fractions if the patient has a preserved performance status in order to achieve durable tumor control; however, many patients have performance statuses that fluctuate.4 There are clinical situations where shortened courses of one to five fractions are the best option in highly symptomatic patients. Additionally, when treating metastatic disease—particularly bone metastases—clinical trials have shown no difference in pain reduction with single fraction versus more prolonged treatment courses. The utilization of single-fraction treatments for palliation has been more slowly adopted in the United States, compared with Europe for example, for unclear reasons. I think we should base our decisions on individual patient presentations.
We are using SBRT more frequently for patients with stage IV disease to try to improve progression-free survival based on multiple studies showing improvement in this endpoint. NRG LU002 (NCT03137771) is examining administration of SBRT to the primary and metastatic sites of disease after first-line chemotherapy or immunotherapy, using a hypofractionated approach. I think stage IV palliative radiation therapy is becoming more nuanced than palliative radiation therapy for other disease stages because we are using ablative fractionation regimens to achieve local control of the primary and distant disease sites, if they’re limited, which has been a real change in the field for stage IV lung cancer. As our patients are living longer with more effective systemic therapies, there may be more of a role for radiation to local sites of disease. The data from NRG LU002 and other trials will help us make these determinations.
Immunotherapy and Early-Stage Disease
There are also trials being designed to examine whether immunotherapy after SBRT or SBRT alone is better for patients with early-stage disease who are not surgical candidates. These studies may help to further improve the outcomes of patients who are medically inoperable. Also, there are single institutions at large academic centers that are evaluating the optimal timing of immunotherapy, the optimal radiation therapy fractionation regimen, and biomarkers for the optimal selection of patients who receive immunotherapy with radiation. Overall, this is an amazingly exciting time for thoracic radiation oncologists. Through innovation and collaboration, we’ve made quite a bit of progress in the treatment of lung cancer with radiation therapy, but the field awaits continued improvements. We are certainly on the right track and hope that we can continue to improve the lives of our patients with lung cancer. ✦
References:
1. American Cancer Society. Key Statistics for Lung Cancer. https://www.cancer.org/cancer/small-cell-lung-cancer/about/key-statistics.html. Accessed July 20, 2018.
2. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199.
3. Curran WJ, Jr., Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452-1460.
4. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for nonsmall cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Practical Radiation Oncology. [Epub ahead of print April 2018].