By Kara Nyberg, PhD
Adding sintilimab, an anti-PD-1 antibody, to first-line platinum-based chemotherapy enhances efficacy in patients with locally advanced or metastatic nonsquamous NSCLC. In the randomized, placebo-controlled, phase III ORIENT-11 trial conducted in China, median progression-free survival (PFS) reached 5.0 months with chemotherapy but improved to 8.9 months when sintilimab was paired with chemotherapy (hazard ratio [HR] 0.482, 95% CI 0.362-0.643]; P < 0.00001), thus meeting the primary endpoint of the trial. In addition, the chemoimmunotherapy combination produced clear-cut improvements in median PFS across nearly all patient subgroups.
According to lead investigator Li Zhang, MD, of the Sun Yat-Sen University Cancer Centre, treatment options are limited for patients with nonsquamous NSCLC lacking sensitive EGFR mutations or ALK rearrangements. “Treatment with an anti-PD-1 monoclonal antibody in combination with chemotherapy may bring a greater survival benefit to this patient population. We are glad to see that these findings from this trial of sintilimab met the predefined primary endpoint in the interim analysis.”
The 397 patients with untreated locally advanced or metastatic nonsquamous NSCLC who participated in ORIENT-11 were randomly assigned in a 2-to-1 ratio to receive treatment with sintilimab or placebo, each in combination with four cycles of pemetrexed and platinum (either cisplatin or carboplatin). This was followed by maintenance treatment with pemetrexed in combination with either sintilimab or placebo. All study participants first underwent stratification by gender (male vs female), platinum agent (cisplatin vs carboplatin), and PD-L1 expression (tumor proportion score < 1% vs ≥ 1%) before randomization.
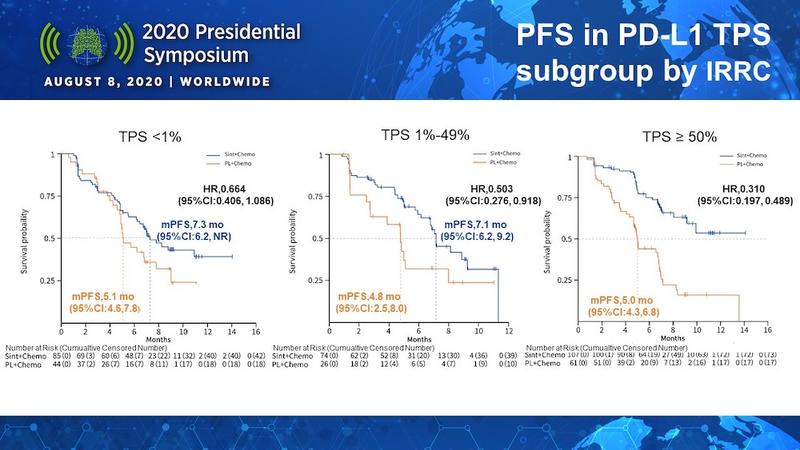
Since the ORIENT-11 population comprised all-comers, a key question of interest was how the level of PD-L1 tumor expression influenced sintilimab efficacy. Dr. Zhang showed data illustrating that sintilimab plus chemotherapy led to greater improvements in median PFS compared with chemotherapy alone as PD-L1 tumor expression increased (Figure). However, even patients with PD-L1 tumor expression below 1% experienced a 2.2-month gain in PFS with sintilimab.
The addition of sintilimab to chemotherapy demonstrated other efficacy improvements as well. Chemoimmunotherapy, when compared to chemotherapy alone, yielded a higher objective response rate (51.9% vs 29.8%), a shorter time to response (1.5 vs 2.6 months), and a longer duration of response (not reached vs 5.5 months). Moreover, even though the final overall survival data are not yet mature and medians have not been reached, the sintilimab-chemotherapy regimen is showing a positive trend in survival rates when measured against chemotherapy (6-month overall survival: 89.6% vs 80.4%; HR 0.609, 95% CI 0.400-0.926; P = 0.01921). This is in spite of the fact that 31% of patients in the placebo arm crossed over to receive sintilimab every 3 weeks for up to 24 months at the time of progression, which may attenuate the survival benefits seen with sintilimab in the investigational arm.
Sintilimab appeared to be fairly well tolerated, with little evidence that it exacerbated the toxicity profile of pemetrexed/platinum. The incidence of overall adverse events (AEs; 99.6% vs 100%), grade 3-5 AEs (61.7% vs 58.8%), serious AEs (28.2% vs 29.8%), AEs leading to death (2.3% vs 6.9%), and AEs leading to treatment discontinuation (6.0% vs 8.4%) were quite similar for the arms that did and did not receive sintilimab. Sintilimab-treated patients did show a slightly higher rate of immune-related AEs in comparison with placebo-treated patients (43.2% vs 36.6%); however, there was no difference between groups in grade 3-5 immune-related AEs (5.6% vs 6.1%).
How does ORIENT-11 data compare to other IO studies? According to Discussant Misako Nagasada, MD, of Karmanos Cancer Institute, the median PFS of 8.9 months appears comparable to other chemoimmunotherapy studies, although longer follow-up will be needed for OS data to mature. In the PD-L1 high (≥ 50%) group, the median PFS was not reached in the ORIENT-11 study, which, based on the hazard ratio of 0.310 and 95% confidence interval, this again appears comparable to other studies, including Keynote-189.
Based on guidance from the ORIENT-11 Independent Data Monitoring Committee, plans are underway to register the sintilimab-chemotherapy combination with the National Medical Products Administration (NMPA). Sintilimab is already approved in China for the treatment of relapsed/refractory Hodgkin lymphoma but is not approved elsewhere.










